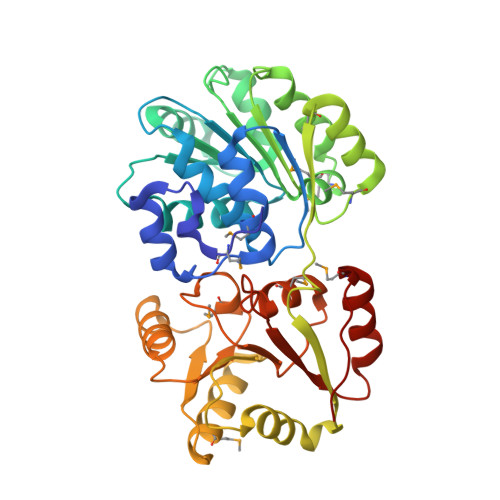
Crystal structure of a DEAD box protein from the hyperthermophile Methanococcus jannaschii.
Story, R.M., Li, H., Abelson, J.N.(2001) Proc Natl Acad Sci U S A 98: 1465-1470
- PubMed: 11171974
- DOI: https://doi.org/10.1073/pnas.98.4.1465
- Primary Citation of Related Structures:
1HV8 - PubMed Abstract:
We have determined the structure of a DEAD box putative RNA helicase from the hyperthermophile Methanococcus jannaschii. Like other helicases, the protein contains two alpha/beta domains, each with a recA-like topology. Unlike other helicases, the protein exists as a dimer in the crystal. Through an interaction that resembles the dimer interface of insulin, the amino-terminal domain's 7-strand beta-sheet is extended to 14 strands across the two molecules. Motifs conserved in the DEAD box family cluster in the cleft between domains, and many of their functions can be deduced by mutational data and by comparison with other helicase structures. Several lines of evidence suggest that motif III Ser-Ala-Thr may be involved in binding RNA.
- California Institute of Technology, Division of Biology 147-75, Pasadena, CA 91125, USA.
Organizational Affiliation: