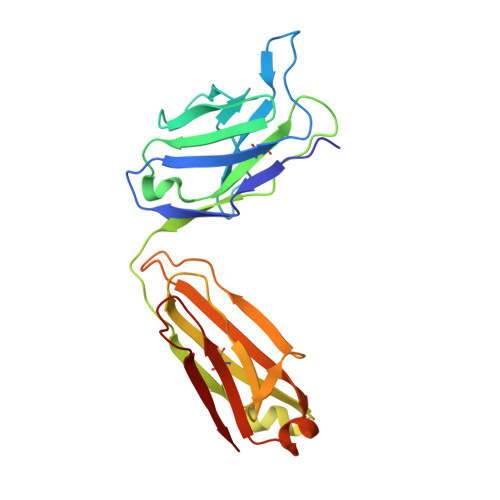
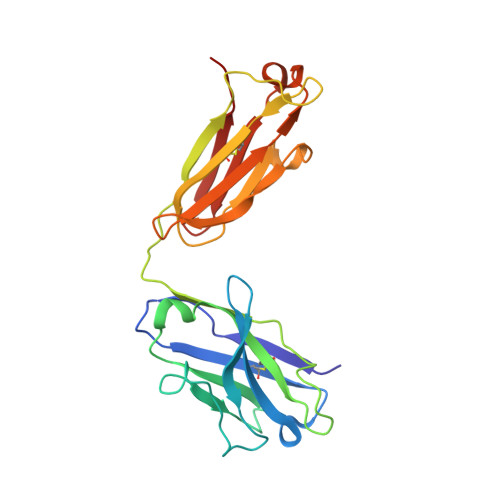
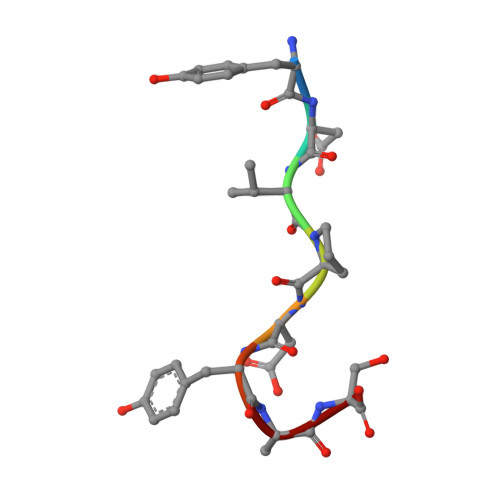
Structural evidence for induced fit as a mechanism for antibody-antigen recognition.
Rini, J.M., Schulze-Gahmen, U., Wilson, I.A.(1992) Science 255: 959-965
- PubMed: 1546293
- DOI: https://doi.org/10.1126/science.1546293
- Primary Citation of Related Structures:
1HIL, 1HIM, 1HIN - PubMed Abstract:
The three-dimensional structure of a specific antibody (Fab 17/9) to a peptide immunogen from influenza virus hemagglutinin [HA1(75-110)] and two independent crystal complexes of this antibody with bound peptide (TyrP100-LeuP108) have been determined by x-ray crystallographic techniques at 2.0 A, 2.9 A, and 3.1 A resolution, respectively. The nonapeptide antigen assumes a type I beta turn in the antibody combining site and interacts primarily with the Fab hypervariable loops L3, H2, and H3. Comparison of the bound and unbound Fab structures shows that a major rearrangement in the H3 loop accompanies antigen binding. This conformational change results in the creation of a binding pocket for the beta turn of the peptide, allowing TyrP105 to be accommodated. The conformation of the peptide bound to the antibody shows similarity to its cognate sequence in the HA1, suggesting a possible mechanism for the cross-reactivity of this Fab with monomeric hemagglutinin. The structures of the free and antigen bound antibodies demonstrate the flexibility of the antibody combining site and provide an example of induced fit as a mechanism for antibody-antigen recognition.
- Department of Molecular Biology, Scripps Research Institute, La Jolla, CA 92037.
Organizational Affiliation: