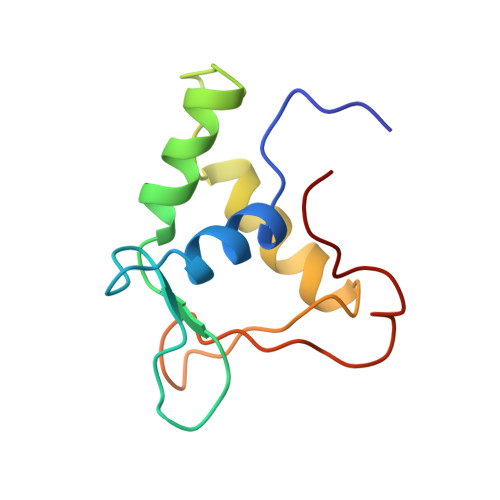
Solution structure of the ets domain of Fli-1 when bound to DNA.
Liang, H., Mao, X., Olejniczak, E.T., Nettesheim, D.G., Yu, L., Meadows, R.P., Thompson, C.B., Fesik, S.W.(1994) Nat Struct Biol 1: 871-875
- PubMed: 7773776
- DOI: https://doi.org/10.1038/nsb1294-871
- Primary Citation of Related Structures:
1FLI - PubMed Abstract:
Members of the ets family of transcription factors share a conserved DNA-binding domain, the ets domain. By using multidimensional NMR, we have determined the structure of the ets domain of human Fli-1 in the DNA-bound form. It consists of three alpha-helices and a four-stranded beta-sheet, similar to structures of the class of helix-turn-helix DNA binding proteins first found in the catabolite activator protein of Escherichia coli. NMR and mutagenesis experiments suggest that in comparison to structurally related proteins, the ets domain uses a new variation of the helix-turn-helix motif for binding to DNA.
- Pharmaceutical Discovery Division, Abbott Laboratories, Abbott Park, Illinois 60064, USA.
Organizational Affiliation: