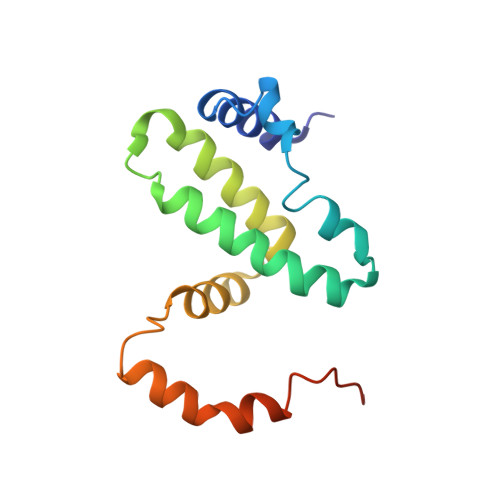
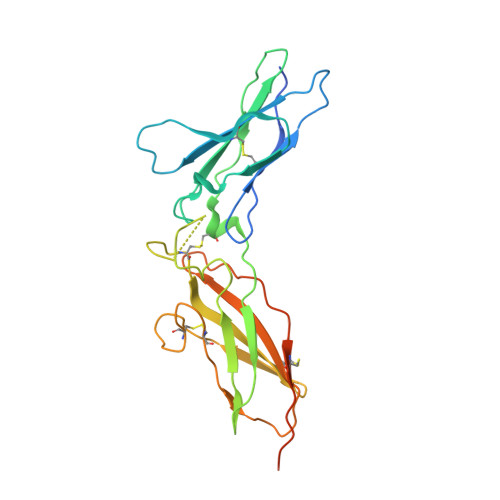
Observation of an unexpected third receptor molecule in the crystal structure of human interferon-gamma receptor complex.
Thiel, D.J., le Du, M.H., Walter, R.L., D'Arcy, A., Chene, C., Fountoulakis, M., Garotta, G., Winkler, F.K., Ealick, S.E.(2000) Structure 8: 927-936
- PubMed: 10986460
- DOI: https://doi.org/10.1016/s0969-2126(00)00184-2
- Primary Citation of Related Structures:
1FG9 - PubMed Abstract:
Molecular interactions among cytokines and cytokine receptors form the basis of many cell-signaling pathways relevant to immune function. Interferon-gamma (IFN-gamma) signals through a multimeric receptor complex consisting of two different but structurally related transmembrane chains: the high-affinity receptor-binding subunit (IFN-gammaRalpha) and a species-specific accessory factor (AF-1 or IFN-gammaRbeta). In the signaling complex, the two receptors probably interact with one another through their extracellular domains. Understanding the atomic interactions of signaling complexes enhances the ability to control and alter cell signaling and also provides a greater understanding of basic biochemical processes. The crystal structure of the complex of human IFN-gamma with the soluble, glycosylated extracellular part of IFN-gammaRalpha has been determined at 2.9 A resolution using multiwavelength anomalous diffraction methods. In addition to the expected 2:1 complex, the crystal structure reveals the presence of a third receptor molecule not directly associated with the IFN-gamma dimer. Two distinct intermolecular contacts, involving the edge strands of the C-terminal domains, are observed between this extra receptor and the 2:1 receptor-ligand complex thereby forming a 3:1 complex. The observed interactions in the 2:1 complex of the high-affinity cell-surface receptor with the IFN-gamma cytokine are similar to those seen in a previously reported structure where the receptor chains were not glycosylated. The formation of beta-sheet packing interactions between pairs of IFN-gammaRalpha receptors in these crystals suggests a possible model for receptor oligomerization of Ralpha and the structurally homologous Rbeta receptors in the fully active IFN-gamma signaling complex.
- Section of Biochemistry, Molecular & Cell Biology, Cornell University, Ithaca, NY 14853, USA.
Organizational Affiliation: