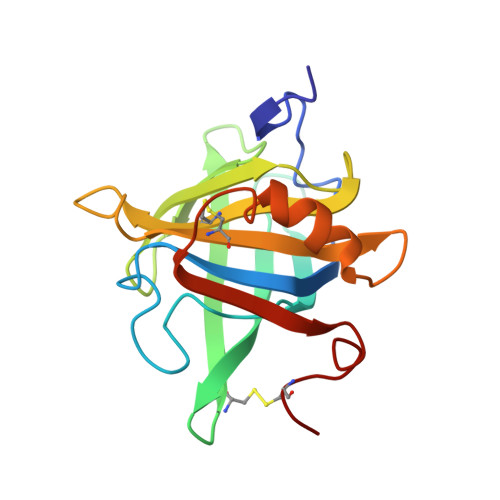
Solution structure and dynamics of bovine beta-lactoglobulin A.
Kuwata, K., Hoshino, M., Forge, V., Era, S., Batt, C.A., Goto, Y.(1999) Protein Sci 8: 2541-2545
- PubMed: 10595563
- DOI: https://doi.org/10.1110/ps.8.11.2541
- Primary Citation of Related Structures:
1CJ5 - PubMed Abstract:
Using heteronuclear NMR spectroscopy, we studied the solution structure and dynamics of bovine beta-lactoglobulin A at pH 2.0 and 45 degrees C, where the protein exists as a monomeric native state. The monomeric NMR structure, comprising an eight-stranded continuous antiparallel beta-barrel and one major alpha-helix, is similar to the X-ray dimeric structure obtained at pH 6.2, including betaI-strand that forms the dimer interface and loop EF that serves as a lid of the interior hydrophobic hole. [1H]-15N NOE revealed that betaF, betaG, and betaH strands buried under the major alpha-helix are rigid on a pico- to nanosecond time scale and also emphasized rapid fluctuations of loops and the N- and C-terminal regions.
- Department of Physiology, School of Medicine, Gifu University, Tsukasamachi, Japan.
Organizational Affiliation: