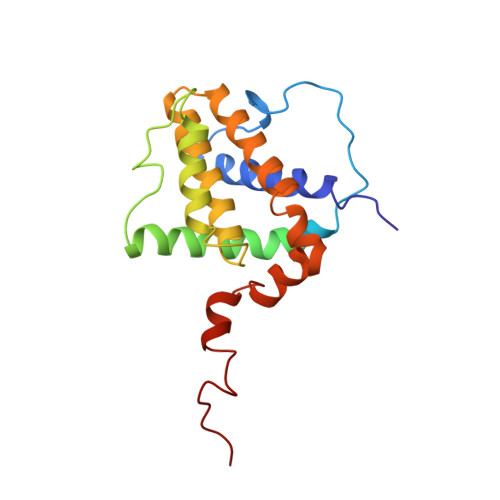
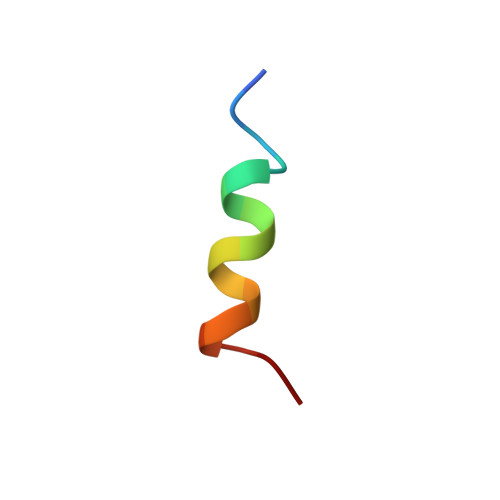
Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis.
Sattler, M., Liang, H., Nettesheim, D., Meadows, R.P., Harlan, J.E., Eberstadt, M., Yoon, H.S., Shuker, S.B., Chang, B.S., Minn, A.J., Thompson, C.B., Fesik, S.W.(1997) Science 275: 983-986
- PubMed: 9020082
- DOI: https://doi.org/10.1126/science.275.5302.983
- Primary Citation of Related Structures:
1BXL - PubMed Abstract:
Heterodimerization between members of the Bcl-2 family of proteins is a key event in the regulation of programmed cell death. The molecular basis for heterodimer formation was investigated by determination of the solution structure of a complex between the survival protein Bcl-xL and the death-promoting region of the Bcl-2-related protein Bak. The structure and binding affinities of mutant Bak peptides indicate that the Bak peptide adopts an amphipathic alpha helix that interacts with Bcl-xL through hydrophobic and electrostatic interactions. Mutations in full-length Bak that disrupt either type of interaction inhibit the ability of Bak to heterodimerize with Bcl-xL.
- Pharmaceutical Discovery Division, Abbott Laboratories, Abbott Park, IL 60064, USA.
Organizational Affiliation: