IRF2BP2 recognizes and bind to a conserved RxSVI sequence motif of protein partners and interacts with ZBTB16 to regulate megakaryocytic differentiation
Wang, G.C., Ding, J.P.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Interferon regulatory factor 2-binding protein 2 | 83 | Homo sapiens | Mutation(s): 0 Gene Names: IRF2BP2 | 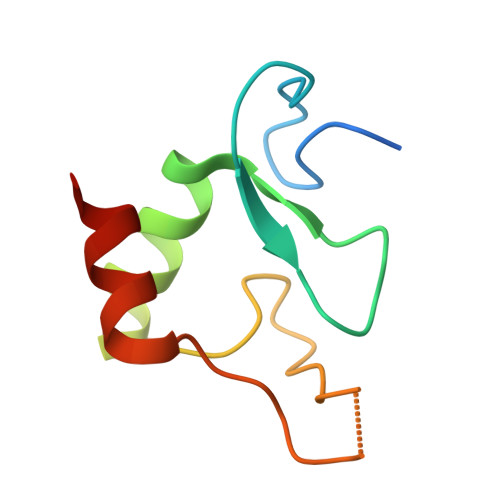 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q7Z5L9 (Homo sapiens) Explore Q7Z5L9 Go to UniProtKB: Q7Z5L9 | |||||
PHAROS: Q7Z5L9 GTEx: ENSG00000168264 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q7Z5L9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Zinc finger and BTB domain-containing protein 16 | 8 | Homo sapiens | Mutation(s): 0 | 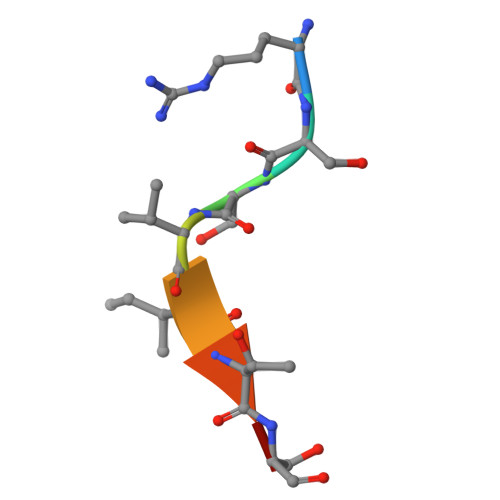 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q05516 (Homo sapiens) Explore Q05516 Go to UniProtKB: Q05516 | |||||
PHAROS: Q05516 GTEx: ENSG00000109906 | |||||
Entity Groups | |||||
| UniProt Group | Q05516 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZN (Subject of Investigation/LOI) Query on ZN | C [auth A], D [auth A], E [auth A] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 47.315 | α = 90 |
| b = 47.315 | β = 90 |
| c = 57.489 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data scaling |
| HKL-2000 | data reduction |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Chinese Academy of Sciences | China | XDB37030305 |