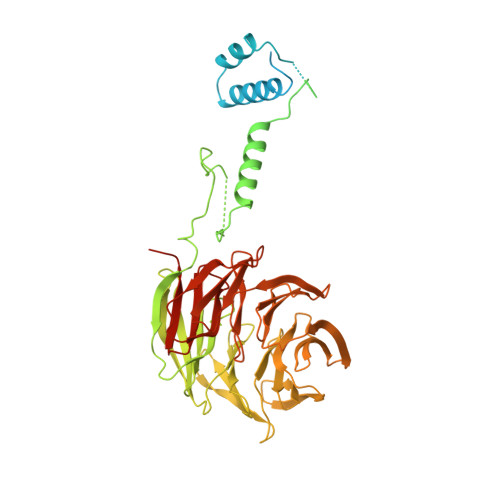
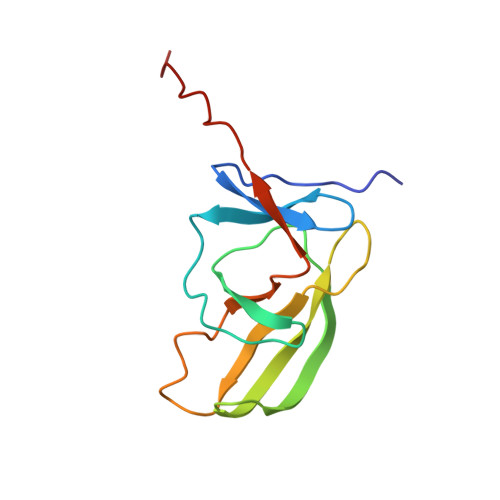
Non-canonical substrate recognition by the human WDR26-CTLH E3 ligase regulates prodrug metabolism.
Gottemukkala, K.V., Chrustowicz, J., Sherpa, D., Sepic, S., Vu, D.T., Karayel, O., Papadopoulou, E.C., Gross, A., Schorpp, K., von Gronau, S., Hadian, K., Murray, P.J., Mann, M., Schulman, B.A., Alpi, A.F.(2024) Mol Cell 84: 1948-1963.e11
- PubMed: 38759627
- DOI: https://doi.org/10.1016/j.molcel.2024.04.014
- Primary Citation of Related Structures:
8QBN, 8QE8 - PubMed Abstract:
The yeast glucose-induced degradation-deficient (GID) E3 ubiquitin ligase forms a suite of complexes with interchangeable receptors that selectively recruit N-terminal degron motifs of metabolic enzyme substrates. The orthologous higher eukaryotic C-terminal to LisH (CTLH) E3 complex has been proposed to also recognize substrates through an alternative subunit, WDR26, which promotes the formation of supramolecular CTLH E3 assemblies. Here, we discover that human WDR26 binds the metabolic enzyme nicotinamide/nicotinic-acid-mononucleotide-adenylyltransferase 1 (NMNAT1) and mediates its CTLH E3-dependent ubiquitylation independently of canonical GID/CTLH E3-family substrate receptors. The CTLH subunit YPEL5 inhibits NMNAT1 ubiquitylation and cellular turnover by WDR26-CTLH E3, thereby affecting NMNAT1-mediated metabolic activation and cytotoxicity of the prodrug tiazofurin. Cryoelectron microscopy (cryo-EM) structures of NMNAT1- and YPEL5-bound WDR26-CTLH E3 complexes reveal an internal basic degron motif of NMNAT1 essential for targeting by WDR26-CTLH E3 and degron mimicry by YPEL5's N terminus antagonizing substrate binding. Thus, our data provide a mechanistic understanding of how YPEL5-WDR26-CTLH E3 acts as a modulator of NMNAT1-dependent metabolism.
Organizational Affiliation:
Department of Molecular Machines and Signaling, Max Planck Institute of Biochemistry, Martinsried 82152, Germany; TUM School of Natural Sciences, Technical University, Munich 85748, Germany.