Bacteriophage origin of some minimal ATP-dependent DNA ligases: a new structure from Burkholderia pseudomallei with striking similarity to Chlorella virus ligase.
Pan, J., Lian, K., Sarre, A., Leiros, H.S., Williamson, A.(2021) Sci Rep 11: 18693-18693
- PubMed: 34548548
- DOI: https://doi.org/10.1038/s41598-021-98155-w
- Primary Citation of Related Structures:
7OBN - PubMed Abstract:
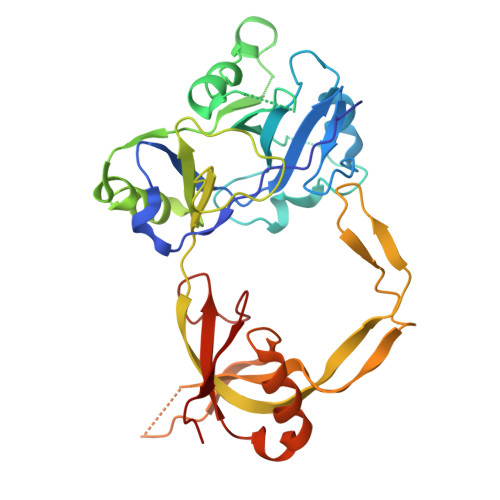
DNA ligases, the enzymes responsible for joining breaks in the phosphodiester backbone of DNA during replication and repair, vary considerably in size and structure. The smallest members of this enzyme class carry out their functions with pared-down protein scaffolds comprising only the core catalytic domains. Here we use sequence similarity network analysis of minimal DNA ligases from all biological super kingdoms, to investigate their evolutionary origins, with a particular focus on bacterial variants. This revealed that bacterial Lig C sequences cluster more closely with Eukaryote and Archaeal ligases, while bacterial Lig E sequences cluster most closely with viral sequences. Further refinement of the latter group delineates a cohesive cluster of canonical Lig E sequences that possess a leader peptide, an exclusively bacteriophage group of T7 DNA ligase homologs and a group with high similarity to the Chlorella virus DNA ligase which includes both bacterial and viral enzymes. The structure and function of the bacterially-encoded Chlorella virus homologs were further investigated by recombinantly producing and characterizing, the ATP-dependent DNA ligase from Burkholderia pseudomallei as well as determining its crystal structure in complex with DNA. This revealed that the enzyme has similar activity characteristics to other ATP-dependent DNA ligases, and significant structural similarity to the eukaryotic virus Chlorella virus including the positioning and DNA contacts of the binding latch region. Analysis of the genomic context of the B. pseudomallei ATP-dependent DNA ligase indicates it is part of a lysogenic bacteriophage present in the B. pseudomallei chromosome representing one likely entry point for the horizontal acquisition of ATP-dependent DNA ligases by bacteria.
Organizational Affiliation:
School of Science, University of Waikato, Hamilton, 3240, New Zealand.