Structural basis of seamless excision and specific targeting by piggyBac transposase
Chen, Q., Luo, W., Veach, R.A., Hickman, A.B., Wilson, M.H., Dyda, F.(2020) Nat Commun 11: 3446-3446
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2020) Nat Commun 11: 3446-3446
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Transposase | A [auth C], B [auth D] | 594 | Trichoplusia ni | Mutation(s): 1 | 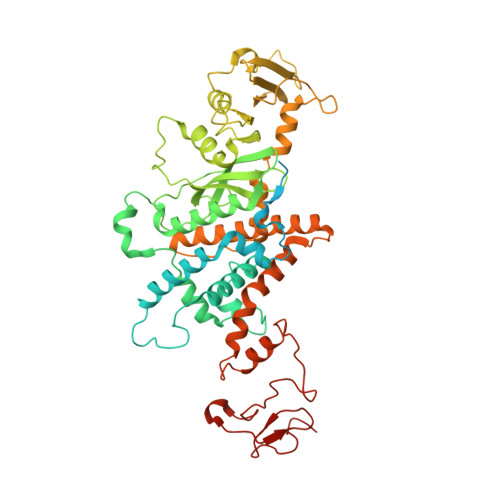 |
UniProt | |||||
Find proteins for Q283G1 (Trichoplusia ni) Explore Q283G1 Go to UniProtKB: Q283G1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q283G1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (37-MER) | C [auth E], F [auth J] | 37 | Trichoplusia ni | 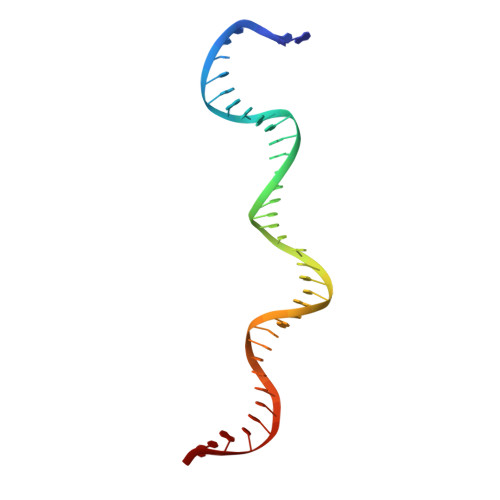 | |
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (47-MER) | D [auth F], G [auth K] | 47 | Trichoplusia ni | 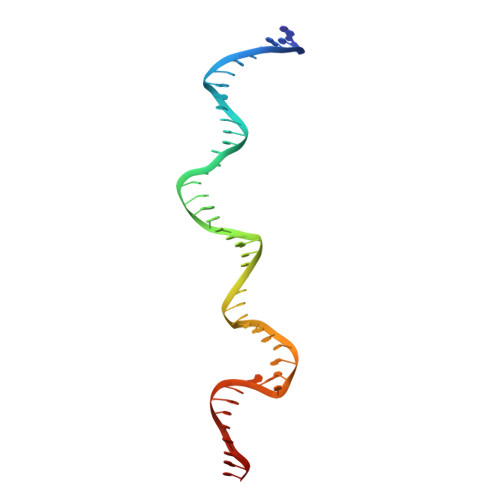 | |
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (tDNA) | E [auth G], H [auth L] | 8 | Trichoplusia ni | 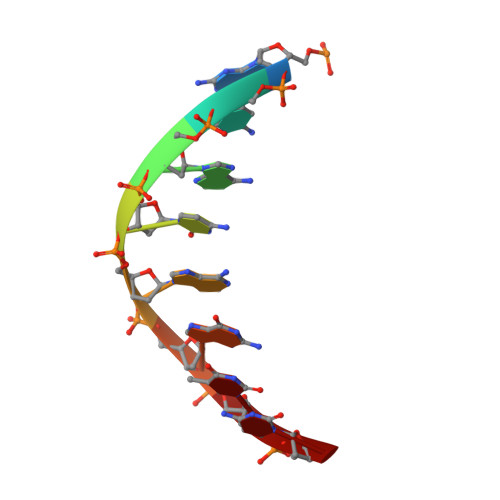 | |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZN Query on ZN | I [auth C], J [auth C], M [auth D], N [auth D] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| CA Query on CA | K [auth C], L [auth C], O [auth D], P [auth D] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | RELION | 3.0 |
| MODEL REFINEMENT | Rosetta |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease (NIH/NIDDK) | United States | DK093660 |