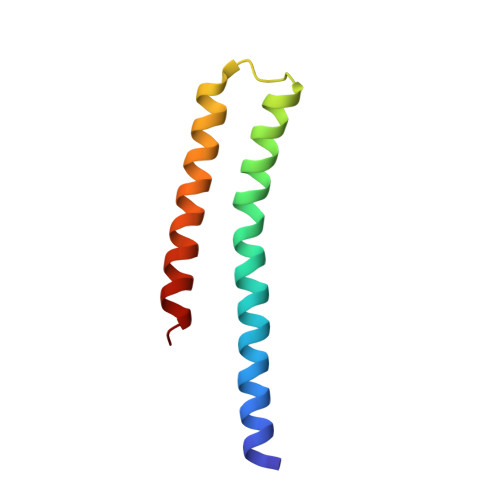
Asymmetric Structure of the Dimerization Domain of PhoR, a Sensor Kinase Important for the Virulence of Mycobacterium tuberculosis.
Xing, D., Ryndak, M.B., Wang, L., Kolesnikova, I., Smith, I., Wang, S.(2017) ACS Omega 2: 3509-3517
- PubMed: 28782049
- DOI: https://doi.org/10.1021/acsomega.7b00612
- Primary Citation of Related Structures:
5UKV, 5UKY - PubMed Abstract:
The PhoP-PhoR two-component system is essential for the virulence of Mycobacterium tuberculosis ( Mtb ) and therefore represents a potential target for developing novel antituberculosis therapies. However, little is known about the mechanism by which this two-component system regulates the virulence. In this study, we demonstrated that a phoR mutant Mtb strain has phenotypes similar to those of a phoP mutant, suggesting that PhoP and PhoR work in the same pathway to regulate Mtb virulence. We determined the structure of the dimerization and histidine phosphotransfer (DHp) domain of PhoR to a 1.9 Å resolution. The structure revealed that the DHp domain is a dimer. Each subunit consists of two antiparallel α helices connected by a loop of five residues. The two subunits of the dimer fold into a four-helical bundle with a continuous hydrophobic core. The topology of the four-helical bundle is identical to the histidine kinases that are known to have a cis-autophosphorylation mechanism, suggesting that PhoR is likely to autophosphorylate in cis. The dimer is asymmetric, with one subunit having a greater bending angle than the other at the highly conserved proline residue five-residues downstream of the phosphorylation site histidine. This structural asymmetry of the dimer suggests the flexibility of the PhoR DHp domain, which is likely to be important for the signal transduction mechanism in controlling the autophosphorylation and phosphotransfer reactions and communicating with the upstream structure.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, Maryland 20814, United States.