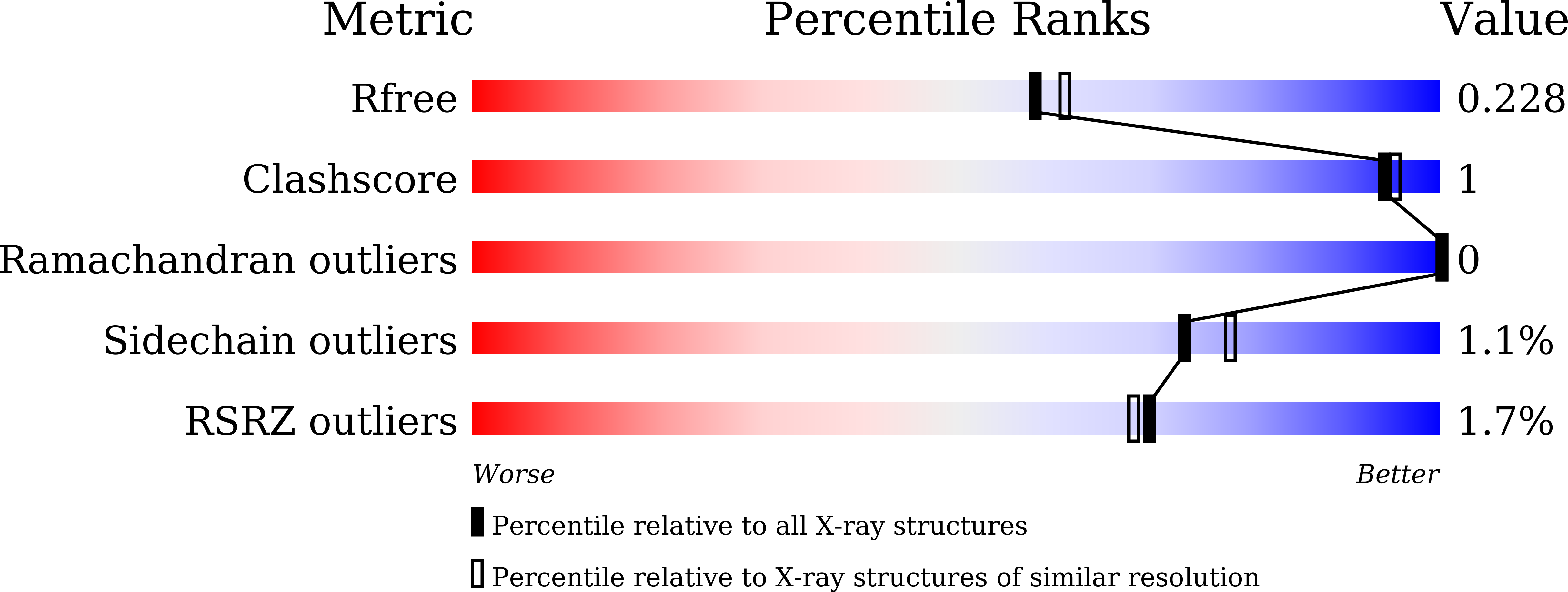
Crystal structures of highly simplified BPTIs provide insights into hydration-driven increase of unfolding enthalpy
Islam, M.M., Yohda, M., Kidokoro, S., Kuroda, Y.(2017) Sci Rep 7: 41205-41205
- PubMed: 28266637
- DOI: https://doi.org/10.1038/srep41205
- Primary Citation of Related Structures:
5JB4, 5JB5, 5JB6, 5JB7 - PubMed Abstract:
We report a thermodynamic and structural analysis of six extensively simplified bovine pancreatic trypsin inhibitor (BPTI) variants containing 19-24 alanines out of 58 residues. Differential scanning calorimetry indicated a two-state thermal unfolding, typical of a native protein with densely packed interior. Surprisingly, increasing the number of alanines induced enthalpy stabilization, which was however over-compensated by entropy destabilization. X-ray crystallography indicated that the alanine substitutions caused the recruitment of novel water molecules facilitating the formation of protein-water hydrogen bonds and improving the hydration shells around the alanine's methyl groups, both of which presumably contributed to enthalpy stabilization. There was a strong correlation between the number of water molecules and the thermodynamic parameters. Overall, our results demonstrate that, in contrast to our initial expectation, a protein sequence in which over 40% of the residues are alanines can retain a densely packed structure and undergo thermal denaturation with a large enthalpy change, mainly contributed by hydration.
Organizational Affiliation:
Department of Biotechnology and Life Science, Tokyo University of Agriculture and Technology, 2-24-16 Nakamachi, Koganei-shi, Tokyo 184-8588, Japan.