Differential function of lip residues in the mechanism and biology of an anthrax hemophore.
Ekworomadu, M.T., Poor, C.B., Owens, C.P., Balderas, M.A., Fabian, M., Olson, J.S., Murphy, F., Balkabasi, E., Honsa, E.S., He, C., Goulding, C.W., Maresso, A.W.(2012) PLoS Pathog 8: e1002559-e1002559
- PubMed: 22412371
- DOI: https://doi.org/10.1371/journal.ppat.1002559
- Primary Citation of Related Structures:
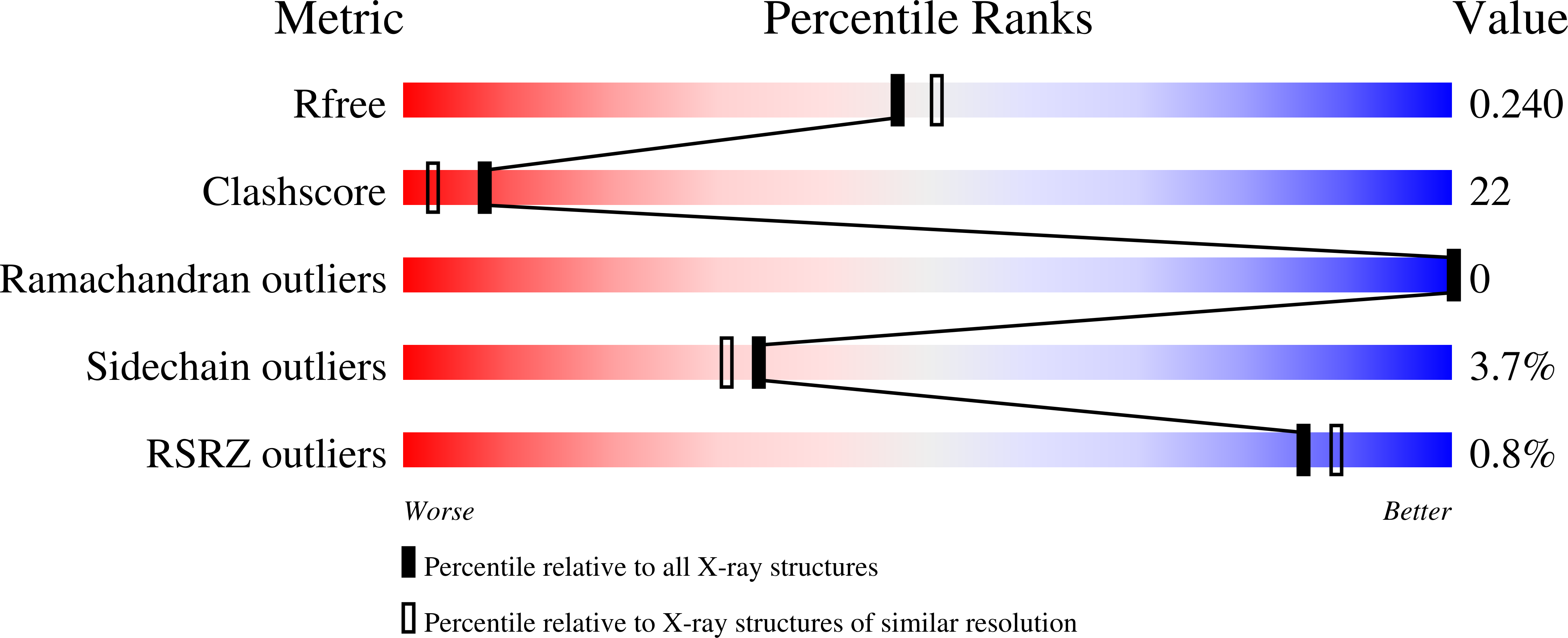
3SIK, 3SZ6 - PubMed Abstract:
To replicate in mammalian hosts, bacterial pathogens must acquire iron. The majority of iron is coordinated to the protoporphyrin ring of heme, which is further bound to hemoglobin. Pathogenic bacteria utilize secreted hemophores to acquire heme from heme sources such as hemoglobin. Bacillus anthracis, the causative agent of anthrax disease, secretes two hemophores, IsdX1 and IsdX2, to acquire heme from host hemoglobin and enhance bacterial replication in iron-starved environments. Both proteins contain NEAr-iron Transporter (NEAT) domains, a conserved protein module that functions in heme acquisition in Gram-positive pathogens. Here, we report the structure of IsdX1, the first of a Gram-positive hemophore, with and without bound heme. Overall, IsdX1 forms an immunoglobin-like fold that contains, similar to other NEAT proteins, a 3(10)-helix near the heme-binding site. Because the mechanistic function of this helix in NEAT proteins is not yet defined, we focused on the contribution of this region to hemophore and NEAT protein activity, both biochemically and biologically in cultured cells. Site-directed mutagenesis of amino acids in and adjacent to the helix identified residues important for heme and hemoglobin association, with some mutations affecting both properties and other mutations affecting only heme stabilization. IsdX1 with mutations that reduced the ability to associate with hemoglobin and bind heme failed to restore the growth of a hemophore-deficient strain of B. anthracis on hemoglobin as the sole iron source. These data indicate that not only is the 3(10)-helix important for NEAT protein biology, but also that the processes of hemoglobin and heme binding can be both separate as well as coupled, the latter function being necessary for maximal heme-scavenging activity. These studies enhance our understanding of NEAT domain and hemophore function and set the stage for structure-based inhibitor design to block NEAT domain interaction with upstream ligands.
Organizational Affiliation:
Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.