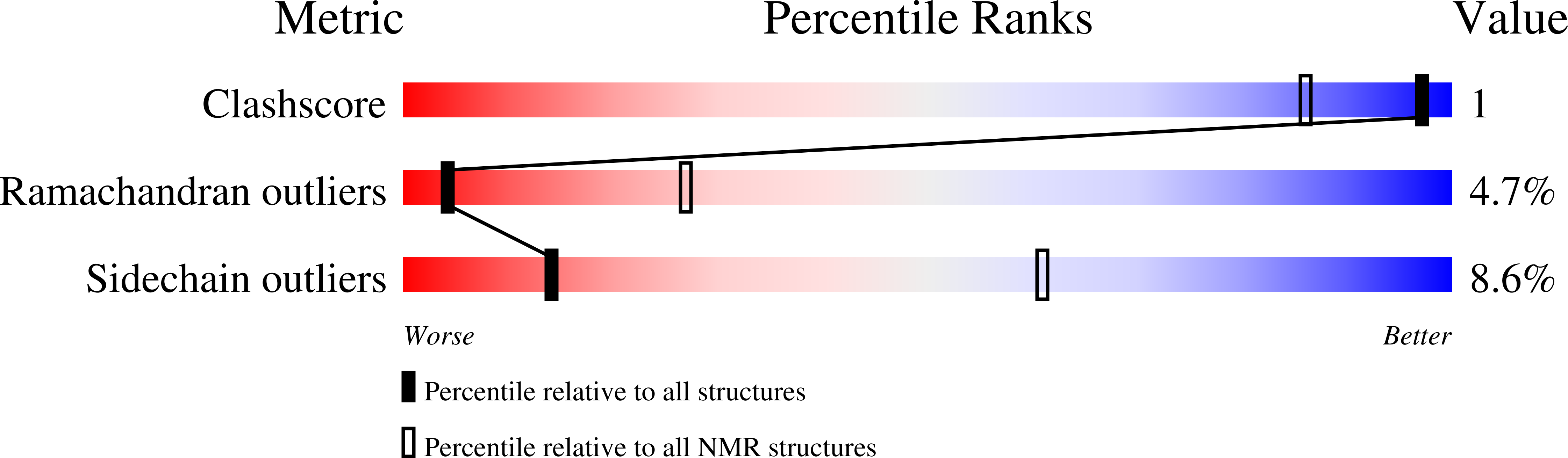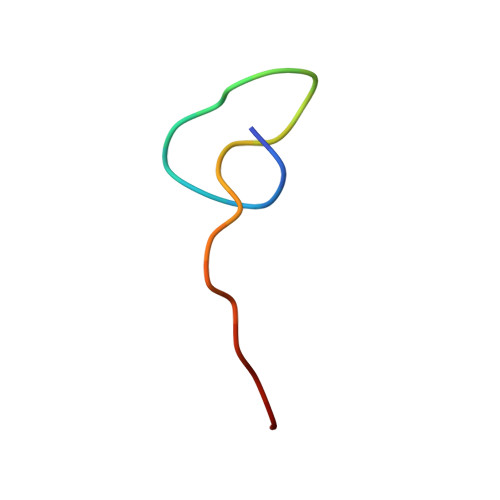Construction of Lasso Peptide Fusion Proteins.
Zong, C., Maksimov, M.O., Link, A.J.(2016) ACS Chem Biol 11: 61-68
- PubMed: 26492187
- DOI: https://doi.org/10.1021/acschembio.5b00745
- Primary Citation of Related Structures:
2N68 - PubMed Abstract:
Lasso peptides are a family of ribosomally synthesized and post-translationally modified peptides (RiPPs) typified by an isopeptide-bonded macrocycle between the peptide N-terminus and an aspartate or glutamate side chain. The C-terminal portion of the peptide threads through the N-terminal macrocycle to give the characteristic lasso fold. Because of the inherent stability, both proteolytic and often thermal, of lasso peptides, we became interested in whether proteins could be fused to the free C-terminus of lasso peptides. Here, we demonstrate fusion of two model proteins, the artificial leucine zipper A1 and the superfolder variant of GFP, to the C-terminus of the lasso peptide astexin-1. Successful lasso cyclization of the N-terminus of these fusion proteins requires a flexible linker in between the C-terminus of the lasso peptide and the N-terminus of the protein of interest. The ability to fuse lasso peptides to a protein of interest is an important step toward phage and bacterial display systems for the high-throughput screening of lasso peptide libraries for new functions.
Organizational Affiliation:
Department of Chemistry, Princeton University , Princeton, New Jersey 08544, United States.