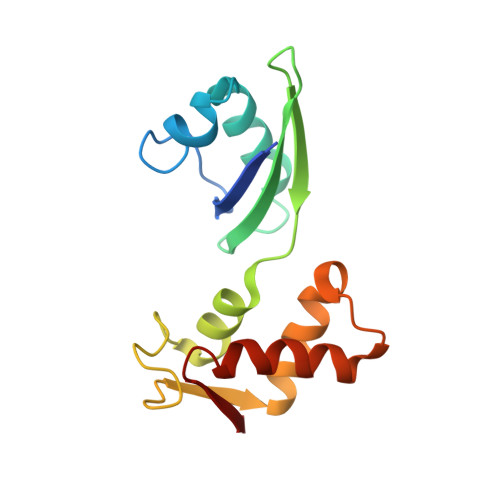
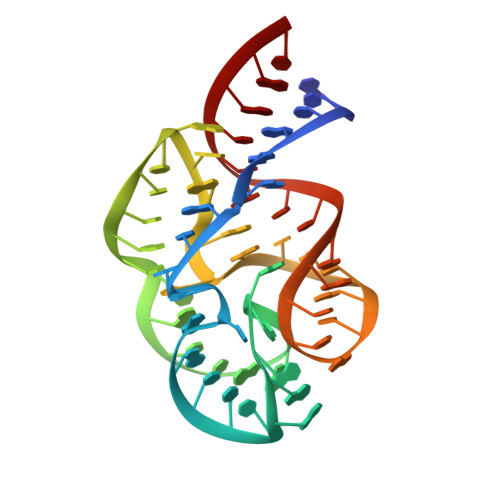
A detailed view of a ribosomal active site: the structure of the L11-RNA complex.
Wimberly, B.T., Guymon, R., McCutcheon, J.P., White, S.W., Ramakrishnan, V.(1999) Cell 97: 491-502
- PubMed: 10338213
- DOI: https://doi.org/10.1016/s0092-8674(00)80759-x
- Primary Citation of Related Structures:
1MMS - PubMed Abstract:
We report the crystal structure of a 58 nucleotide fragment of 23S ribosomal RNA bound to ribosomal protein L11. This highly conserved ribonucleoprotein domain is the target for the thiostrepton family of antibiotics that disrupt elongation factor function. The highly compact RNA has both familiar and novel structural motifs. While the C-terminal domain of L11 binds RNA tightly, the N-terminal domain makes only limited contacts with RNA and is proposed to function as a switch that reversibly associates with an adjacent region of RNA. The sites of mutations conferring resistance to thiostrepton and micrococcin line a narrow cleft between the RNA and the N-terminal domain. These antibiotics are proposed to bind in this cleft, locking the putative switch and interfering with the function of elongation factors.
Organizational Affiliation:
Department of Biochemistry, University of Utah School of Medicine, Salt Lake City 84132, USA.