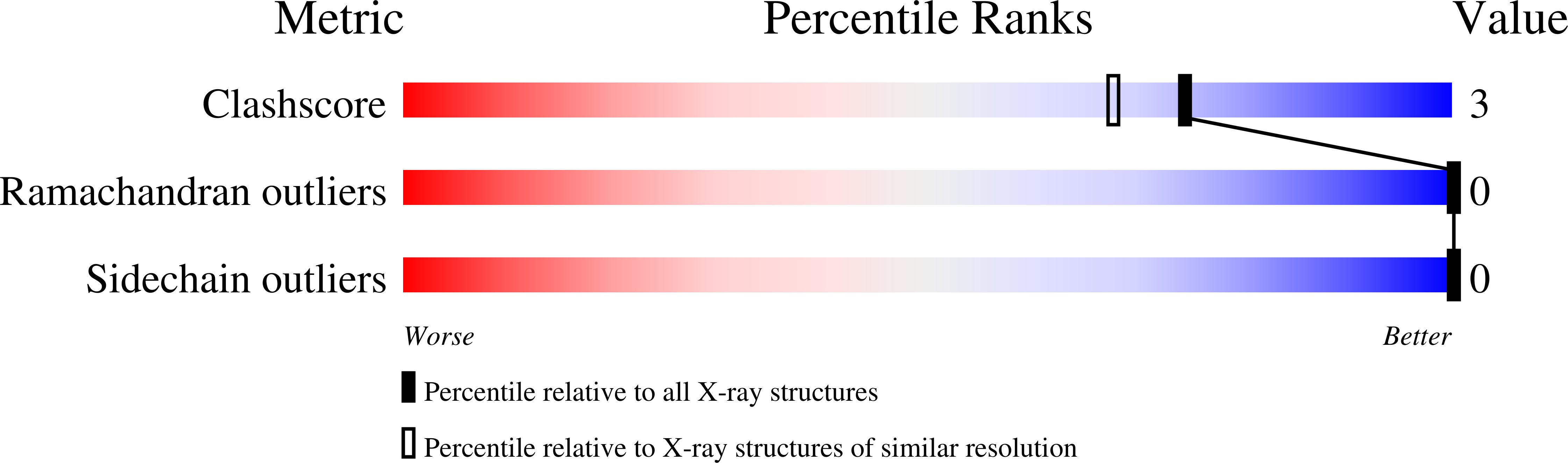Noncysteinyl coordination to the [4Fe-4S]2+ cluster of the DNA repair adenine glycosylase MutY introduced via site-directed mutagenesis. Structural characterization of an unusual histidinyl-coordinated cluster.
Messick, T.E., Chmiel, N.H., Golinelli, M.P., Langer, M.R., Joshua-Tor, L., David, S.S.(2002) Biochemistry 41: 3931-3942
- PubMed: 11900536
- DOI: https://doi.org/10.1021/bi012035x
- Primary Citation of Related Structures:
1KQJ - PubMed Abstract:
The Escherichia coli DNA repair enzyme MutY plays an important role in the recognition and repair of 7,8-dihydro-8-oxo-2'-deoxyguanosine-2'-deoxyadenosine (OG*A) mismatches in DNA. MutY prevents DNA mutations caused by the misincorporation of A opposite OG by catalyzing the deglycosylation of the aberrant adenine. MutY is representative of a unique subfamily of DNA repair enzymes that also contain a [4Fe-4S]2+ cluster, which has been implicated in substrate recognition. Previously, we have used site-directed mutagenesis to individually replace the cysteine ligands to the [4Fe-4S]2+ cluster of E. coli MutY with serine, histidine, or alanine. These experiments suggested that histidine coordination to the iron-sulfur cluster may be accommodated in MutY at position 199. Purification and enzymatic analysis of C199H and C199S forms indicated that these forms behave nearly identical to the WT enzyme. Furthermore, introduction of the C199H mutation in a truncated form of MutY (C199HT) allowed for crystallization and structural characterization of the modified [4Fe-4S] cluster coordination. The C199HT structure showed that histidine coordinated to the iron cluster although comparison to the structure of the WT truncated enzyme indicated that the occupancy of iron at the modified position had been reduced to 60%. Electron paramagnetic resonance (EPR) spectroscopy on samples of C199HT indicates that a significant percentage (15-30%) of iron clusters were of the [3Fe-4S]1+ form. Oxidation of the C199HT enzyme with ferricyanide increases the amount of the 3Fe cluster by approximately 2-fold. Detailed kinetic analysis on samples containing a mixture of [3Fe-4S]1+ and [4Fe-4S]2+ forms indicated that the reactivity of the [3Fe-4S]1+ C199HT enzyme does not differ significantly from that of the WT truncated enzyme. The relative resistance of the [4Fe-4S]2+ cluster toward oxidation, as well as the retention of activity of the [3Fe-4S]1+ form, may be an important aspect of the role of MutY in repair of DNA damage resulting from oxidative stress.
Organizational Affiliation:
Department of Chemistry, University of Utah, Salt Lake City, Utah 84112, USA.