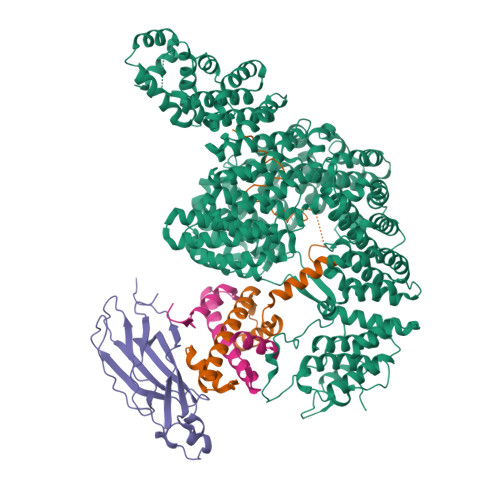
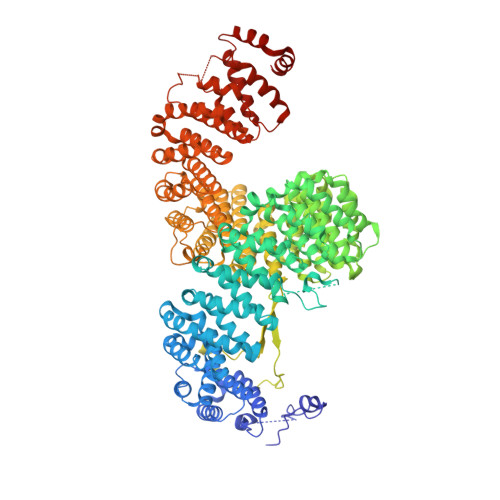
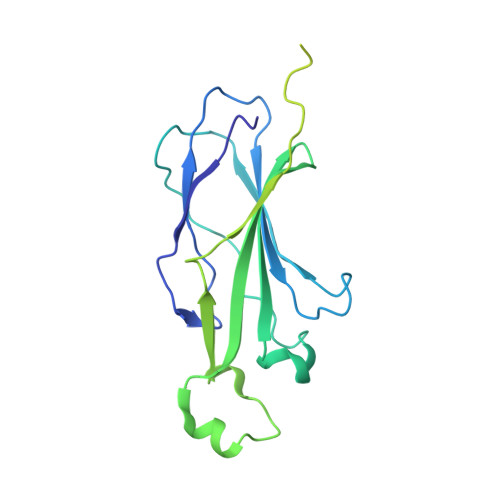
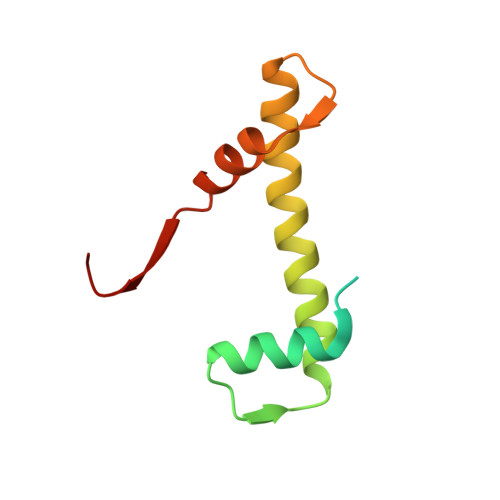
Structure of IMPORTIN-4 bound to the H3-H4-ASF1 histone-histone chaperone complex.
Bernardes, N.E., Fung, H.Y.J., Li, Y., Chen, Z., Chook, Y.M.(2022) Proc Natl Acad Sci U S A 119: e2207177119-e2207177119
- PubMed: 36103578
- DOI: https://doi.org/10.1073/pnas.2207177119
- Primary Citation of Related Structures:
7UNK, 8DYO - PubMed Abstract:
IMPORTIN-4, the primary nuclear import receptor of core histones H3 and H4, binds the H3-H4 dimer and histone chaperone ASF1 prior to nuclear import. However, how H3-H3-ASF1 is recognized for transport cannot be explained by available crystal structures of IMPORTIN-4-histone tail peptide complexes. Our 3.5-Å IMPORTIN-4-H3-H4-ASF1 cryoelectron microscopy structure reveals the full nuclear import complex and shows a binding mode different from suggested by previous structures. The N-terminal half of IMPORTIN-4 clamps the globular H3-H4 domain and H3 αN helix, while its C-terminal half binds the H3 N-terminal tail weakly; tail contribution to binding energy is negligible. ASF1 binds H3-H4 without contacting IMPORTIN-4. Together, ASF1 and IMPORTIN-4 shield nucleosomal H3-H4 surfaces to chaperone and import it into the nucleus where RanGTP binds IMPORTIN-4, causing large conformational changes to release H3-H4-ASF1. This work explains how full-length H3-H4 binds IMPORTIN-4 in the cytoplasm and how it is released in the nucleus.
Organizational Affiliation:
Department of Pharmacology, University of Texas Southwestern Medical Center, Dallas, TX 75390.