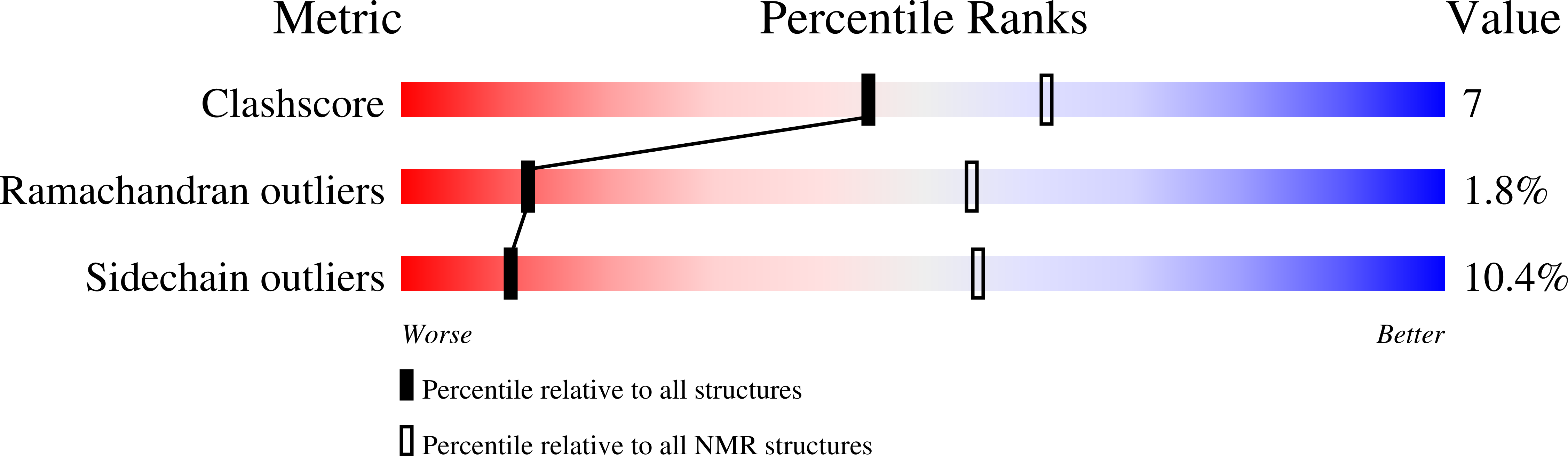Dissociation of the trimeric gp41 ectodomain at the lipid-water interface suggests an active role in HIV-1 Env-mediated membrane fusion.
Roche, J., Louis, J.M., Grishaev, A., Ying, J., Bax, A.(2014) Proc Natl Acad Sci U S A 111: 3425-3430
- PubMed: 24550514
- DOI: https://doi.org/10.1073/pnas.1401397111
- Primary Citation of Related Structures:
2MK3 - PubMed Abstract:
The envelope glycoprotein gp41 mediates the process of membrane fusion that enables entry of the HIV-1 virus into the host cell. The actual fusion process involves a switch from a homotrimeric prehairpin intermediate conformation, consisting of parallel coiled-coil helices, to a postfusion state where the ectodomains are arranged as a trimer of helical hairpins, adopting a six-helix bundle (6HB) state. Here, we show by solution NMR spectroscopy that a water-soluble 6HB gp41 ectodomain binds to zwitterionic detergents that contain phosphocholine or phosphatidylcholine head groups and phospholipid vesicles that mimic T-cell membrane composition. Binding results in the dissociation of the 6HB and the formation of a monomeric state, where its two α-helices, N-terminal heptad repeat (NHR) and C-terminal heptad repeat (CHR), become embedded in the lipid-water interface of the virus and host cell. The atomic structure of the gp41 ectodomain monomer, based on NOE distance restraints and residual dipolar couplings, shows that the NHR and CHR helices remain mostly intact, but they completely lose interhelical contacts. The high affinity of the ectodomain helices for phospholipid surfaces suggests that unzippering of the prehairpin intermediate leads to a state where the NHR and CHR helices become embedded in the host cell and viral membranes, respectively, thereby providing a physical force for bringing these membranes into close juxtaposition before actual fusion.
Organizational Affiliation:
Laboratory of Chemical Physics, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892.