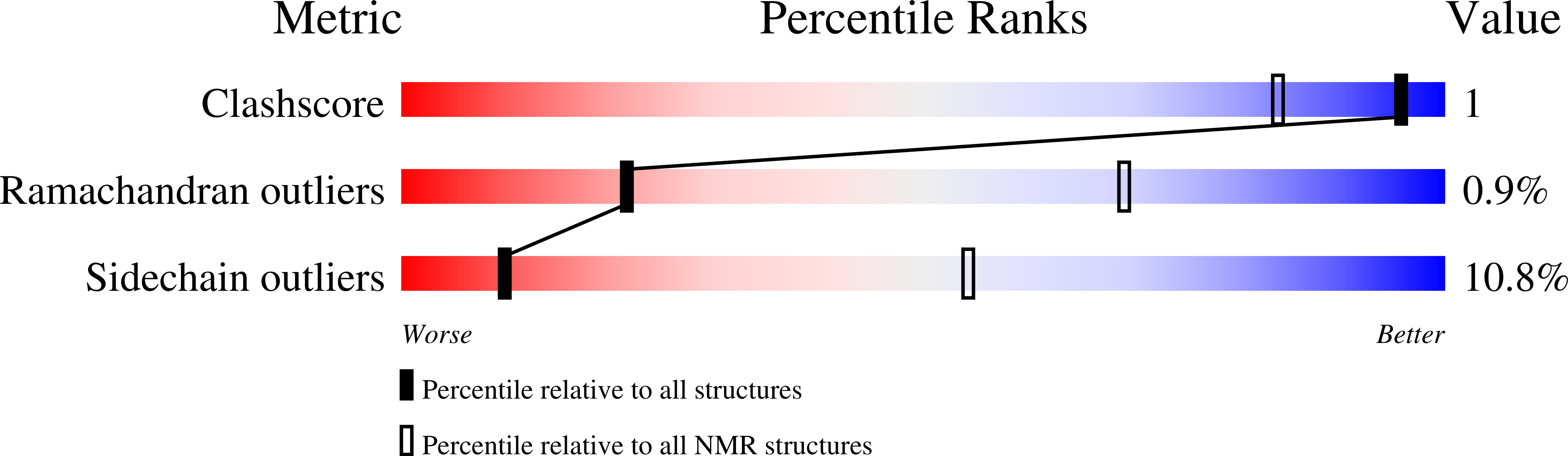Dimerisation of the UBA Domain of p62 Inhibits Ubiquitin Binding and Regulates NF-kappaB Signalling
Long, J., Garner, T.P., Pandya, M.J., Craven, C.J., Chen, P., Shaw, B., Williamson, M.P., Layfield, R., Searle, M.S.(2009) J Mol Biol
- PubMed: 19931284
- DOI: https://doi.org/10.1016/j.jmb.2009.11.032
- Primary Citation of Related Structures:
2KNV - PubMed Abstract:
The ubiquitin (Ub)-binding p62 scaffold protein (encoded by the SQSTM1 gene) regulates a diverse range of signalling pathways leading to activation of the nuclear factor kappa B (NF-kappaB) family of transcription factors and is an important regulator of macroautophagy. Mutations within the gene encoding p62 are commonly found in patients with Paget's disease of bone and largely cluster within the C-terminal ubiquitin-associated (UBA) domain, impairing its ability to bind Ub, resulting in dysregulated NF-kappaB signalling. However, precisely how Ub-binding is regulated at the molecular level is unclear. NMR relaxation dispersion experiments, coupled with concentration-dependent NMR, CD, isothermal titration calorimetry and fluorescence kinetic measurements, reveal that the p62 UBA domain forms a highly stable dimer (K(dim) approximately 4-12 microM at 298 K). NMR analysis shows that the dimer interface partially occludes the Ub-binding surface, particularly at the C-terminus of helix 3, making UBA dimerisation and Ub-binding mutually exclusive processes. Somewhat unusually, the monomeric UBA appears to be the biologically active form and the dimer appears to be the inactive one. Engineered point mutations in loop 1 (E409K and G410K) are shown to destabilise the dimer interface, lead to a higher proportion of the bound monomer and, in NF-kappaB luciferase reporter assays, are associated with reduced NF-kappaB activity compared with wt-p62.
Organizational Affiliation:
Centre for Biomolecular Sciences, University Park, Nottingham NG7 2RD, UK.