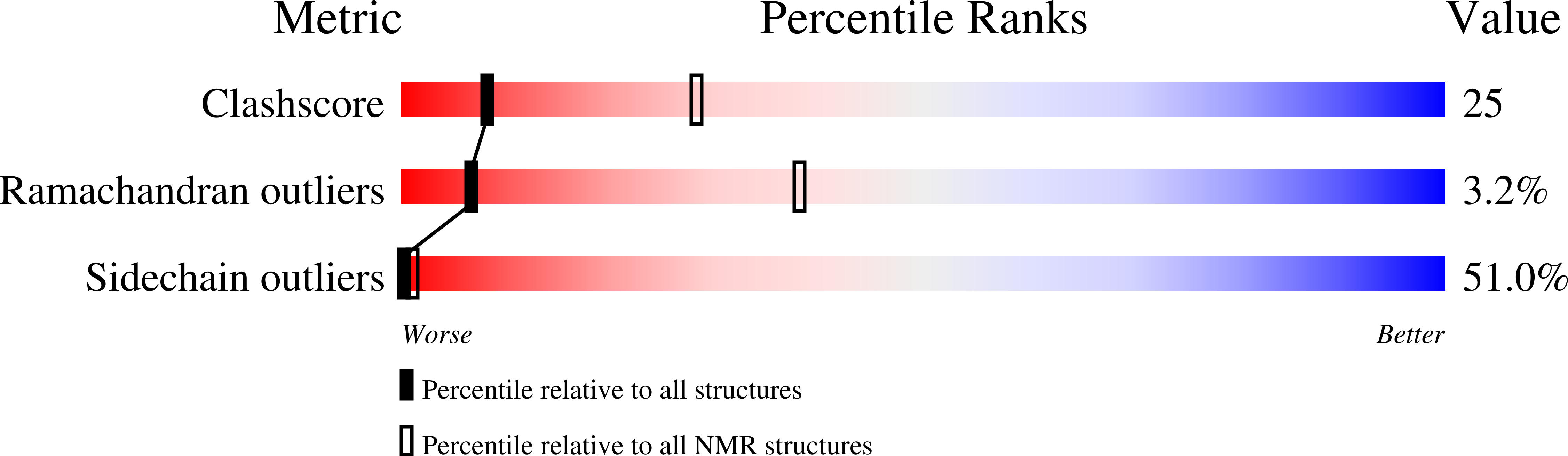Structure and positioning comparison of two variants of penetratin in two different membrane mimicking systems by NMR
Lindberg, M., Biverstahl, H., Graslund, A., Maler, L.(2003) Eur J Biochem 270: 3055-3063
- PubMed: 12846839
- DOI: https://doi.org/10.1046/j.1432-1033.2003.03685.x
- Primary Citation of Related Structures:
1OMQ - PubMed Abstract:
The Antennapedia homeodomain protein of Drosophila has the ability to penetrate biological membranes and the third helix of this protein, residues 43-58, known as penetratin (RQIKIWFQNRRMKWKK-amide) has the same translocating properties as the entire protein. The variant, RQI KIFFQNRRMKFKK-amide, here called penetratin (W48F,W56F) does not have the same ability. We have determined a solution structure of penetratin and investigated the position of both peptides in negatively charged bicelles. A helical structure is seen for residues Lys46 through Met54. The secondary structure of the variant penetratin(W48F,W56F) in bicelles appears to be very similar. Paramagnetic spin-label studies and analysis of NOEs between penetratin and the phospholipids show that penetratin is located within the bicelle surface. Penetratin (W48F,W56F) is also located inside the phospholipid bicelle, however, with its N-terminus more deeply inserted than that of wild-type penetratin. The subtle differences in the way the two peptides interact with a membrane in an equilibrium situation could be important for their translocating ability. As a comparison we have also investigated the secondary structure of penetratin(W48F,W56F) in SDS micelles and the results show that the structure is very similar in SDS and bicelles. In contrast, penetratin(W48F,W56F) and penetratin appear to be located differently in SDS micelles. This clearly shows the importance of using realistic membrane mimetics for investigating peptide-membrane interactions.
Organizational Affiliation:
Department of Biochemistry and Biophysics, The Arrhenius Laboratories, Stockholm University, Sweden.