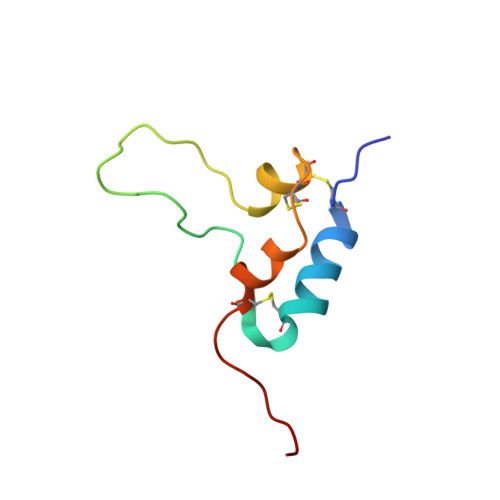
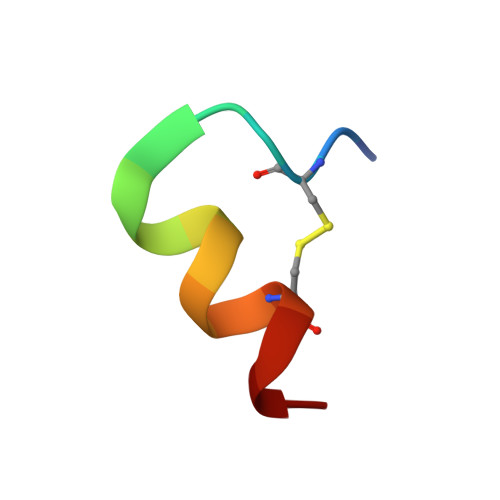
Complex with a Phage Display-Derived Peptide Provides Insight into the Function of Insulin-like Growth Factor I
Schaffer, M.L., Deshayes, K., Nakamura, G., Sidhu, S., Skelton, N.J.(2003) Biochemistry 42: 9324-9334
- PubMed: 12899619
- DOI: https://doi.org/10.1021/bi034386c
- Primary Citation of Related Structures:
1PMX - PubMed Abstract:
The dramatic improvement in the NMR spectra of insulin-like growth factor I (IGF-I) in the presence of a peptide identified from a phage display library has allowed for the first time the determination of a high-resolution solution structure for much of IGF-I. The three helices of IGF-I in this complex have an arrangement similar to that seen in high-resolution crystal structures of IGF-I and insulin, although there are differences in the conformation and precise location of helix 3. A cluster of hydrophobic and basic side chains within the turn-helix motif of the peptide contact a hydrophobic patch on helices 1 and 3 of IGF-I. The importance of this patch for tight binding was verified using alanine scanning mutagenesis of the peptide in two different phage display formats. Consistent with its antagonistic activity, the peptide binds to a region implicated by mutagenesis studies to be important for association with IGF binding proteins (IGFBPs). The ability of the peptide to also inhibit signaling has important implications for the manner in which IGF-I interacts with its receptor. Interestingly, the peptide uses the same binding site as detergent and a fragment of IGFBP-5 identified in other IGF-I complexes. The ligand-induced structural variability of helix 3 in these complexes suggests that exchange between such conformations may be the source of the dynamic nature of free IGF-I and likely has functional significance for the ability of IGF-I to recognize two signaling receptors and six binding proteins with high affinity.
Organizational Affiliation:
Department of Protein Engineering, Genentech, Inc., 1 DNA Way, South San Francisco, California 94080, USA.