Structural basis for the selective addition of an oxygen atom to cyclic ketones by Baeyer-Villiger monooxygenase from Parvibaculum lavamentivorans.
Nguyen, T.D., Choi, G.E., Gu, D.H., Seo, P.W., Kim, J.W., Park, J.B., Kim, J.S.(2019) Biochem Biophys Res Commun 512: 564-570
- PubMed: 30914200
- DOI: https://doi.org/10.1016/j.bbrc.2019.03.114
- Primary Citation of Related Structures:
6JDK - PubMed Abstract:
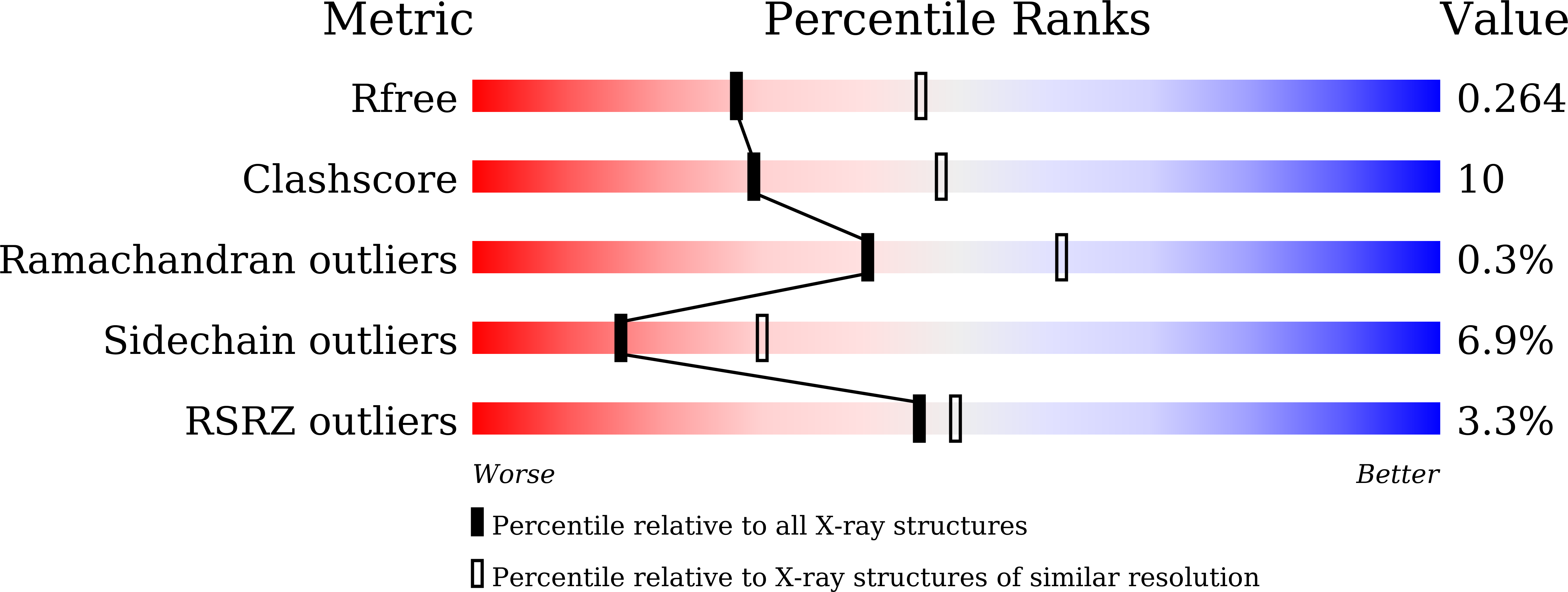
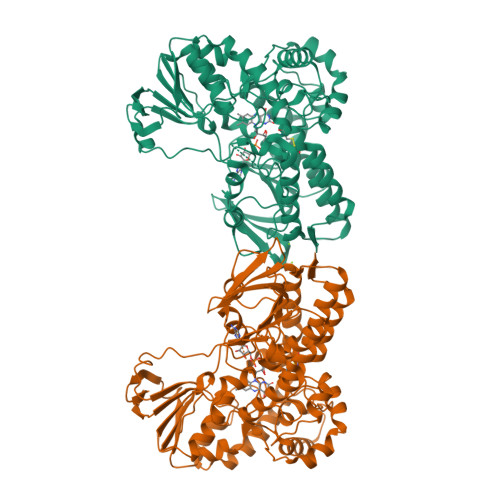
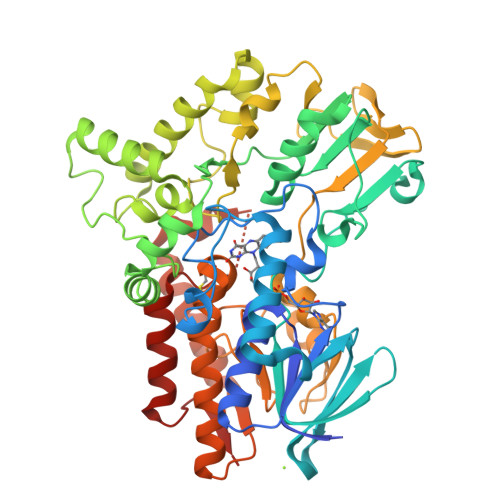
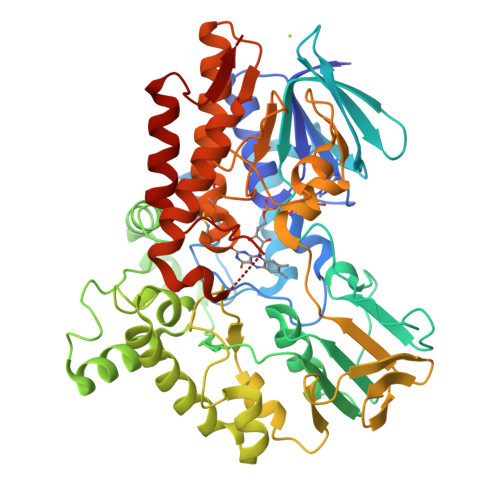
Baeyer-Villiger monooxygenase (BVMO) catalyzes insertion of an oxygen atom into aliphatic or cyclic ketones with high regioselectivity. The BVMOs from Parvibaculum lavamentivorans (BVMO Parvi ) and Oceanicola batsensis (BVMO Ocean ) are interesting because of their homologies, with >40% sequence identity, and reaction with the same cyclic ketones with a methyl moiety to give different products. The revealed BVMO Parvi structure shows that BVMO Parvi forms a two-domain structure like other BVMOs. It has two inserted residues, compared with BVMO Ocean , that form a bulge near the bound flavin adenine dinucleotide in the active site. Furthermore, this bulge is linked to a nearby α-helix via a disulfide bond, probably restricting access of the bulky methyl group of the substrate to this bulge. Another sequence motif at the entrance of the active site (Ala-Ser in BVMO Parvi and Ser-Thr in BVMO Ocean ) allows a large volume in BVMO Parvi . These minute differences may discriminate a substrate orientation in both BVMOs from the initial substrate binding pocket to the final oxygenation site, resulting in the inserted oxygen atom being in different positions of the same substrate.
Organizational Affiliation:
Department of Chemistry, Chonnam National University, Gwangju, 61186, Republic of Korea.