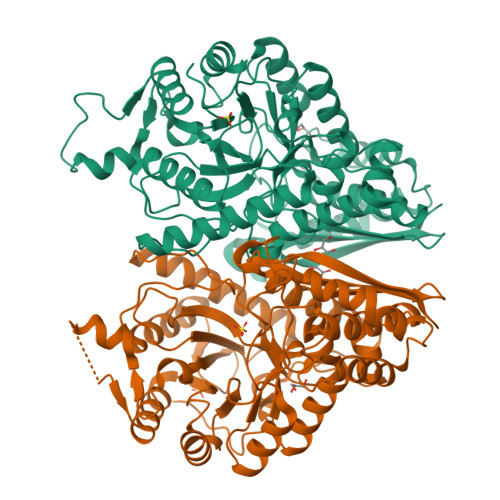
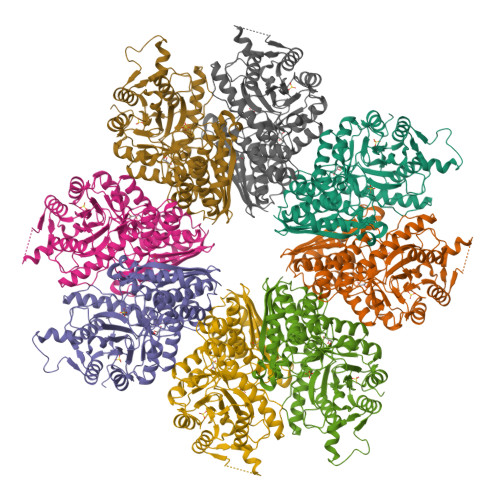
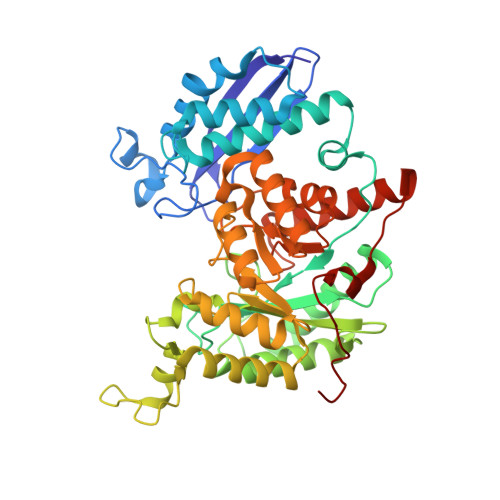
Featured Species-Specific Loops Are Found in the Crystal Structure ofMhpEno, a Cell Surface Adhesin FromMycoplasma hyopneumoniae.
Chen, R., Yu, Y., Feng, Z., Gan, R., Xie, X., Zhang, Z., Xie, Q., Wang, W., Ran, T., Zhang, W., Xiong, Q., Shao, G.(2019) Front Cell Infect Microbiol 9: 209-209
- PubMed: 31263685
- DOI: https://doi.org/10.3389/fcimb.2019.00209
- Primary Citation of Related Structures:
6J36 - PubMed Abstract:
Enolase is an evolutionarily conserved enzyme involved in the processes of glycolysis and gluconeogenesis. Mycoplasma hyopneumoniae belongs to Mycoplasma , whose species are wall-less and among the smallest self-replicating bacteria, and is an important colonizing respiratory pathogen in the pig industry worldwide. Mycoplasma hyopneumoniae enolase ( Mhp Eno) expression is significantly increased after infection and was previously found to be a virulence factor candidate. Our studies show that Mhp Eno is a cell surface-localized protein that can adhere to swine tracheal epithelial cells (STECs). Adhesion to STECs can be specifically inhibited by an Mhp Eno antibody. Mhp Eno can recognize and interact with plasminogen with high affinity. Here, the first crystal structure of the mycoplasmal enolase from Mycoplasma hyopneumoniae was determined. The structure showed unique features of Mhp Eno in the S3/H1, H6/S6, H7/H8, and H13 regions. All of these regions were longer than those of other enolases and were exposed on the Mhp Eno surface, making them accessible to host molecules. These results show that Mhp Eno has specific structural characteristics and acts as a multifunctional adhesin on the Mycoplasma hyopneumoniae cell surface.
Organizational Affiliation:
Key Laboratory of Veterinary Biological Engineering and Technology of Ministry of Agriculture, National Center for Engineering Research of Veterinary Bioproducts, Institute of Veterinary Medicine, Jiangsu Academy of Agricultural Sciences, Nanjing, China.