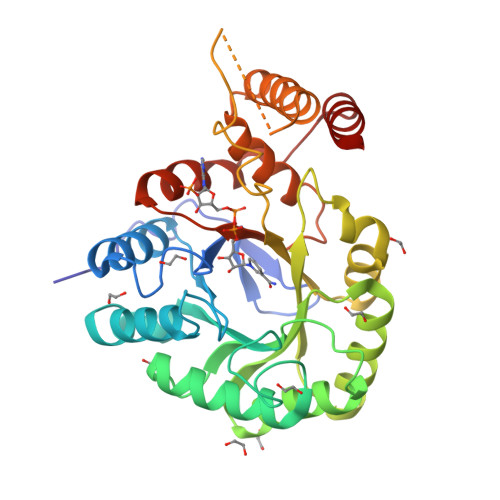
Crystal Structure and Biophysical Analysis of Furfural-Detoxifying Aldehyde Reductase from Clostridium beijerinckii.
Scott, A.F., Cresser-Brown, J., Williams, T.L., Rizkallah, P.J., Jin, Y., Luk, L.Y., Allemann, R.K.(2019) Appl Environ Microbiol 85
- PubMed: 31101612
- DOI: https://doi.org/10.1128/AEM.00978-19
- Primary Citation of Related Structures:
6HG6 - PubMed Abstract:
Many aldehydes, such as furfural, are present in high quantities in lignocellulose lysates and are fermentation inhibitors, which makes biofuel production from this abundant carbon source extremely challenging. Cbei_3974 has recently been identified as an aldo-keto reductase responsible for partial furfural resistance in Clostridium beijerinckii Rational engineering of this enzyme could enhance the furfural tolerance of this organism, thereby improving biofuel yields. We report an extensive characterization of Cbei_3974 and a single-crystal X-ray structure of Cbei_3974 in complex with NADPH at a resolution of 1.75 Å. Docking studies identified residues involved in substrate binding, and an activity screen revealed the substrate tolerance of the enzyme. Hydride transfer, which is partially rate limiting under physiological conditions, occurs from the pro- R hydrogen of NADPH. Enzyme isotope labeling revealed a temperature-independent enzyme isotope effect of unity, indicating that the enzyme does not use dynamic coupling for catalysis and suggesting that the active site of the enzyme is optimally configured for catalysis with the substrate tested. IMPORTANCE Here we report the crystal structure and biophysical properties of an aldehyde reductase that can detoxify furfural, a common inhibitor of biofuel fermentation found in lignocellulose lysates. The data contained here will serve as a guide for protein engineers to develop improved enzyme variants that would impart furfural resistance to the microorganisms used in biofuel production and thus lead to enhanced biofuel yields from this sustainable resource.
Organizational Affiliation:
School of Chemistry, Cardiff University, Cardiff, United Kingdom.