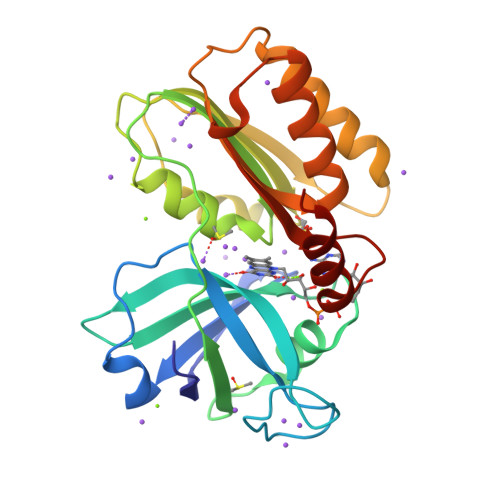
Structure and reactivity of a siderophore-interacting protein from the marine bacteriumShewanellareveals unanticipated functional versatility.
Trindade, I.B., Silva, J.M., Fonseca, B.M., Catarino, T., Fujita, M., Matias, P.M., Moe, E., Louro, R.O.(2019) J Biological Chem 294: 157-167
- PubMed: 30420426
- DOI: https://doi.org/10.1074/jbc.RA118.005041
- Primary Citation of Related Structures:
6GEH - PubMed Abstract:
Siderophores make iron accessible under iron-limited conditions and play a crucial role in the survival of microorganisms. Because of their remarkable metal-scavenging properties and ease in crossing cellular envelopes, siderophores hold great potential in biotechnological applications, raising the need for a deeper knowledge of the molecular mechanisms underpinning the siderophore pathway. Here, we report the structural and functional characterization of a siderophore-interacting protein from the marine bacterium Shewanella frigidimarina NCIBM400 (SfSIP). SfSIP is a flavin-containing ferric-siderophore reductase with FAD- and NAD(P)H-binding domains that have high homology with other characterized SIPs. However, we found here that it mechanistically departs from what has been described for this family of proteins. Unlike other FAD-containing SIPs, SfSIP did not discriminate between NADH and NADPH. Furthermore, SfSIP required the presence of the Fe 2+ -scavenger, ferrozine, to use NAD(P)H to drive the reduction of Shewanella -produced hydroxamate ferric-siderophores. Additionally, this is the first SIP reported that also uses a ferredoxin as electron donor, and in contrast to NAD(P)H, its utilization did not require the mediation of ferrozine, and electron transfer occurred at fast rates. Finally, FAD oxidation was thermodynamically coupled to deprotonation at physiological pH values, enhancing the solubility of ferrous iron. On the basis of these results and the location of the SfSIP gene downstream of a sequence for putative binding of aerobic respiration control protein A (ArcA), we propose that SfSIP contributes an additional layer of regulation that maintains cellular iron homeostasis according to environmental cues of oxygen availability and cellular iron demand.
Organizational Affiliation:
Instituto de Tecnologia Química e Biológica António Xavier (ITQB-NOVA), Universidade Nova de Lisboa, Av. da República (EAN), 2780-157 Oeiras, Portugal.