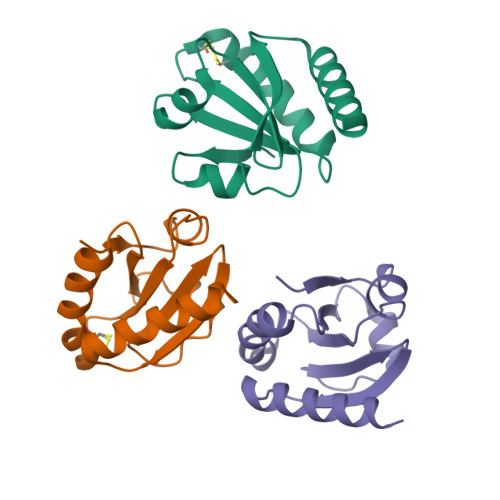
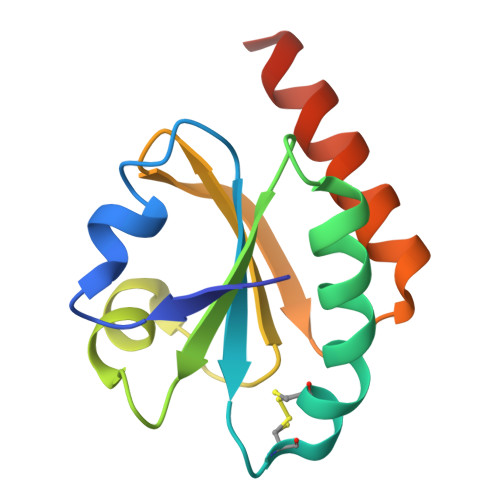
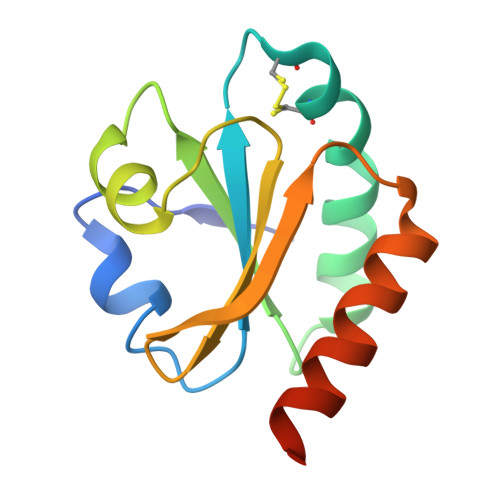
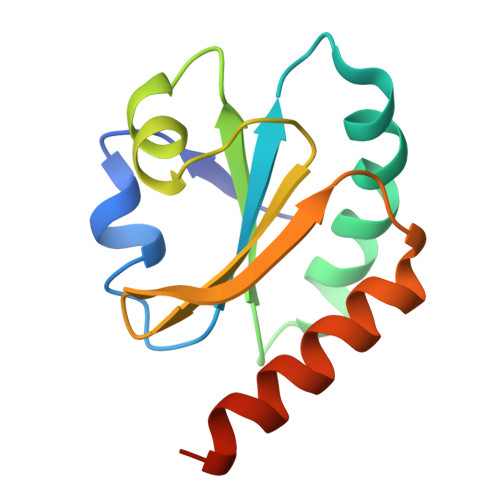
An essential thioredoxin is involved in the control of the cell cycle in the bacterium
Goemans, C.V., Beaufay, F., Wahni, K., Van Molle, I., Messens, J., Collet, J.F.(2018) J Biological Chem 293: 3839-3848
- PubMed: 29367337
- DOI: https://doi.org/10.1074/jbc.RA117.001042
- Primary Citation of Related Structures:
6ESX - PubMed Abstract:
Thioredoxins (Trxs) are antioxidant proteins that are conserved among all species. These proteins have been extensively studied and perform reducing reactions on a broad range of substrates. Here, we identified Caulobacter crescentus Trx1 (CCNA_03653; Cc Trx1) as an oxidoreductase that is involved in the cell cycle progression of this model bacterium and is required to sustain life. Intriguingly, the abundance of Cc Trx1 varies throughout the C. crescentus cell cycle: although the expression of Cc Trx1 is induced in stalked cells, right before DNA replication initiation, Cc Trx1 is actively degraded by the ClpXP protease in predivisional cells. Importantly, we demonstrated that regulation of the abundance of Cc Trx1 is crucial for cell growth and survival as modulating Cc Trx1 levels leads to cell death. Finally, we also report a comprehensive biochemical and structural characterization of this unique and essential Trx. The requirement to precisely control the abundance of Cc Trx1 for cell survival underlines the importance of redox control for optimal cell cycle progression in C. crescentus .
Organizational Affiliation:
From WELBIO, Avenue Hippocrate 75, 1200 Brussels, Belgium, camille.goemans@uclouvain.be.