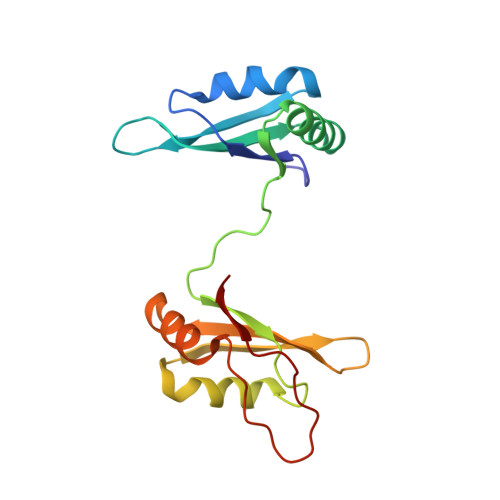
Hinge like domain motion facilitates human RBMS1 protein binding to proto-oncogene c-myc promoter.
Aggarwal, P., Bhavesh, N.S.(2021) Nucleic Acids Res 49: 5943-5955
- PubMed: 33999211
- DOI: https://doi.org/10.1093/nar/gkab363
- Primary Citation of Related Structures:
6M75, 7C36 - PubMed Abstract:
DNA binding proteins recognize DNA specifically or non-specifically using direct and indirect readout mechanisms like sliding, hopping, and diffusion. However, a common difficulty in explicitly elucidating any particular mechanism of site-specific DNA-protein recognition is the lack of knowledge regarding target sequences and inadequate account of non-specific interactions, in general. Here, we decipher the structural basis of target search performed by the key regulator of expression of c-myc proto-oncogene, the human RBMS1 protein. In this study, we have shown the structural reorganization of this multi-domain protein required for recognizing the specific c-myc promoter sequence. The results suggest that a synergy between structural re-organization and thermodynamics is necessary for the recognition of target sequences. The study presents another perspective of looking at the DNA-protein interactions.
Organizational Affiliation:
Transcription Regulation group, International Centre for Genetic Engineering and Biotechnology (ICGEB), Aruna Asaf Ali Marg, New Delhi 110067, India.