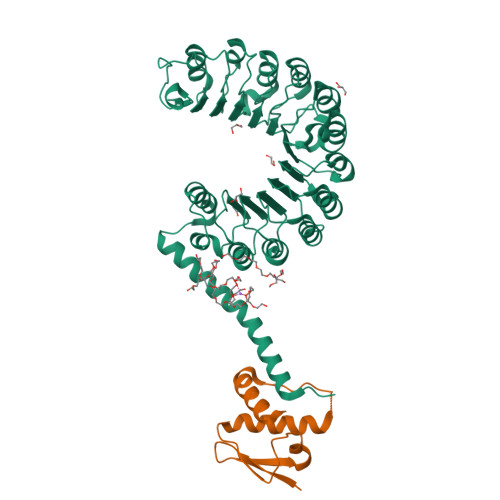
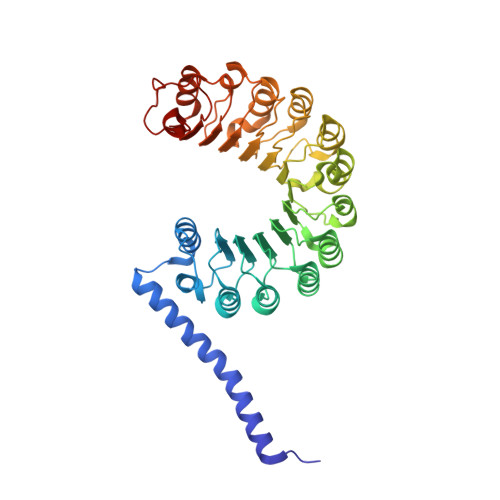
Crystal structure of the yeast Rad7-Elc1 complex and assembly of the Rad7-Rad16-Elc1-Cul3 complex.
Liu, L., Huo, Y., Li, J., Jiang, T.(2019) DNA Repair (Amst) 77: 1-9
- PubMed: 30840920
- DOI: https://doi.org/10.1016/j.dnarep.2019.02.012
- Primary Citation of Related Structures:
5ZB2 - PubMed Abstract:
Nucleotide excision repair (NER) is a versatile system that deals with various bulky and helix-distorting DNA lesions caused by UV and environmental mutagens. Based on how lesion recognition occurs, NER has been separated into global genome repair (GGR) and transcription-coupled repair (TCR). The yeast Rad7-Rad16 complex is indispensable for the GGR sub-pathway. Rad7-Rad16 binds to UV-damaged DNA in a synergistic fashion with Rad4, the main lesion recognizer, to achieve efficient recognition of lesions. In addition, Rad7-Rad16 associates with Elc1 and Cul3 to form an EloC-Cul-SOCS-box (ECS)-type E3 ubiquitin ligase complex that ubiquitinates Rad4 in response to UV radiation. However, the structure and architecture of the Rad7-Rad16-Elc1-Cul3 complex remain unsolved. Here, we determined the structure of the Rad7-Elc1 complex and revealed key interaction regions responsible for the formation of the Rad7-Rad16-Elc1-Cul3 complex. These results provide new insights into the assembly of the Rad7-Rad16-Elc1-Cul3 complex and structural framework for further studies.
Organizational Affiliation:
National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China; School of Medicine, Sun Yat-Sen University, Guangzhou 510275, China.