Unprecedented pathway of reducing equivalents in a diflavin-linked disulfide oxidoreductase.
Buey, R.M., Arellano, J.B., Lopez-Maury, L., Galindo-Trigo, S., Velazquez-Campoy, A., Revuelta, J.L., de Pereda, J.M., Florencio, F.J., Schurmann, P., Buchanan, B.B., Balsera, M.(2017) Proc Natl Acad Sci U S A 114: 12725-12730
- PubMed: 29133410
- DOI: https://doi.org/10.1073/pnas.1713698114
- Primary Citation of Related Structures:
5JRI, 5K0A, 5N0J, 5ODE - PubMed Abstract:
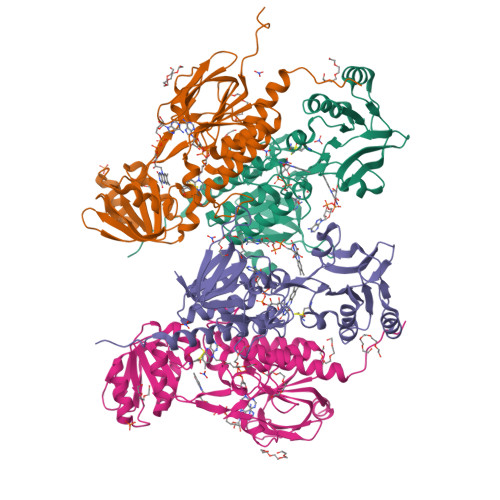
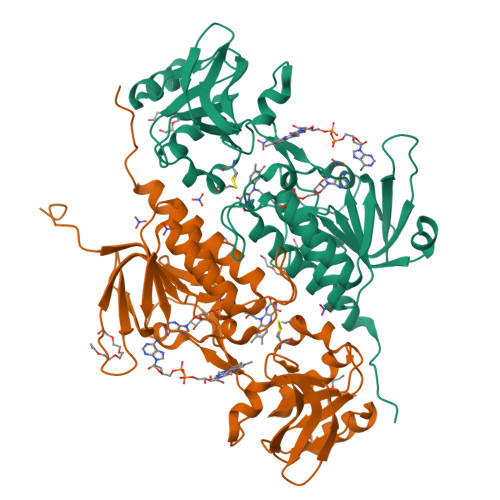
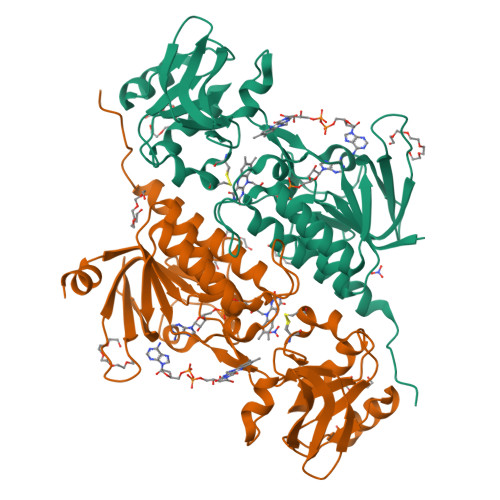
Flavoproteins participate in a wide variety of physiologically relevant processes that typically involve redox reactions. Within this protein superfamily, there exists a group that is able to transfer reducing equivalents from FAD to a redox-active disulfide bridge, which further reduces disulfide bridges in target proteins to regulate their structure and function. We have identified a previously undescribed type of flavin enzyme that is exclusive to oxygenic photosynthetic prokaryotes and that is based on the primary sequence that had been assigned as an NADPH-dependent thioredoxin reductase (NTR). However, our experimental data show that the protein does not transfer reducing equivalents from flavins to disulfides as in NTRs but functions in the opposite direction. High-resolution structures of the protein from Gloeobacter violaceus and Synechocystis sp. PCC6803 obtained by X-ray crystallography showed two juxtaposed FAD molecules per monomer in redox communication with an active disulfide bridge in a variant of the fold adopted by NTRs. We have tentatively named the flavoprotein "DDOR" (diflavin-linked disulfide oxidoreductase) and propose that its activity is linked to a thiol-based transfer of reducing equivalents in bacterial membranes. These findings expand the structural and mechanistic repertoire of flavoenzymes with oxidoreductase activity and pave the way to explore new protein engineering approaches aimed at designing redox-active proteins for diverse biotechnological applications.
Organizational Affiliation:
Metabolic Engineering Group, Departamento de Microbiología y Genética, Universidad de Salamanca, 37007 Salamanca, Spain.