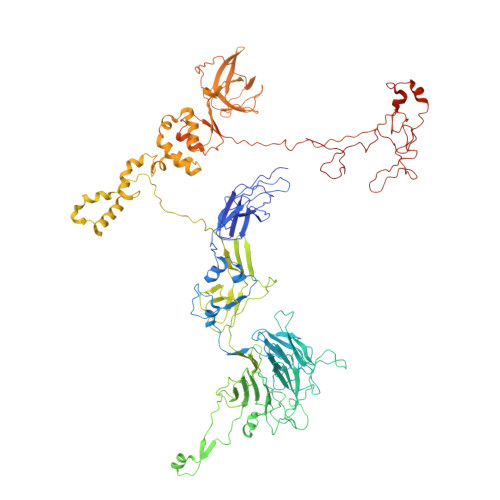
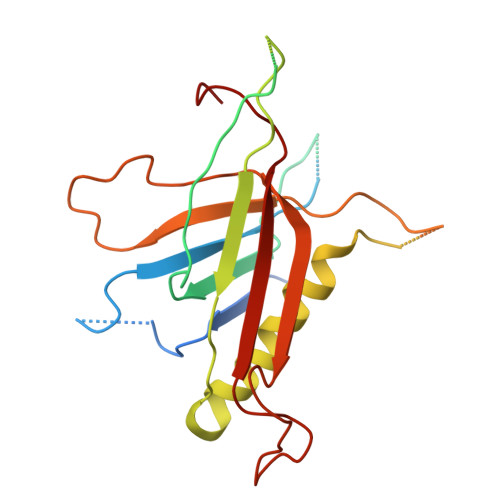
Structure of the T4 baseplate and its function in triggering sheath contraction.
Taylor, N.M., Prokhorov, N.S., Guerrero-Ferreira, R.C., Shneider, M.M., Browning, C., Goldie, K.N., Stahlberg, H., Leiman, P.G.(2016) Nature 533: 346-352
- PubMed: 27193680
- DOI: https://doi.org/10.1038/nature17971
- Primary Citation of Related Structures:
5IV5, 5IV7, 5IW9 - PubMed Abstract:
Several systems, including contractile tail bacteriophages, the type VI secretion system and R-type pyocins, use a multiprotein tubular apparatus to attach to and penetrate host cell membranes. This macromolecular machine resembles a stretched, coiled spring (or sheath) wound around a rigid tube with a spike-shaped protein at its tip. A baseplate structure, which is arguably the most complex part of this assembly, relays the contraction signal to the sheath. Here we present the atomic structure of the approximately 6-megadalton bacteriophage T4 baseplate in its pre- and post-host attachment states and explain the events that lead to sheath contraction in atomic detail. We establish the identity and function of a minimal set of components that is conserved in all contractile injection systems and show that the triggering mechanism is universally conserved.
- École Polytechnique Fédérale de Lausanne (EPFL), BSP-415, 1015 Lausanne, Switzerland.
Organizational Affiliation: