In vitro and in vivo characterization of the multiple isoforms of Schistosoma mansoni hypoxanthine-guanine phosphoribosyltransferases.
Romanello, L., Zeraik, A.E., de Freitas Fernandes, A., Torini, J.R., Bird, L.E., Nettleship, J.E., Rada, H., Reddivari, Y., Owens, R.J., Serrao, V.H.B., DeMarco, R., Brandao-Neto, J., Pereira, H.D.(2019) Mol Biochem Parasitol 229: 24-34
- PubMed: 30772423
- DOI: https://doi.org/10.1016/j.molbiopara.2019.02.005
- Primary Citation of Related Structures:
5IPF - PubMed Abstract:
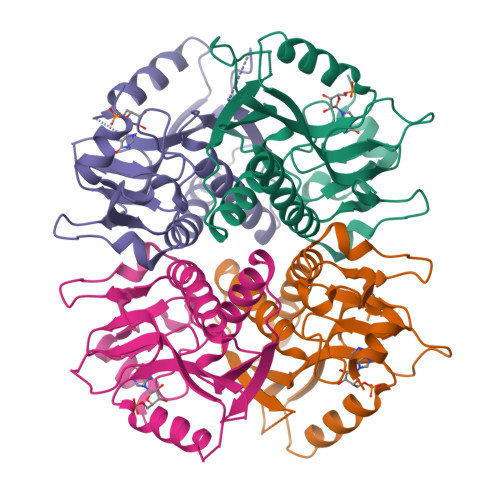
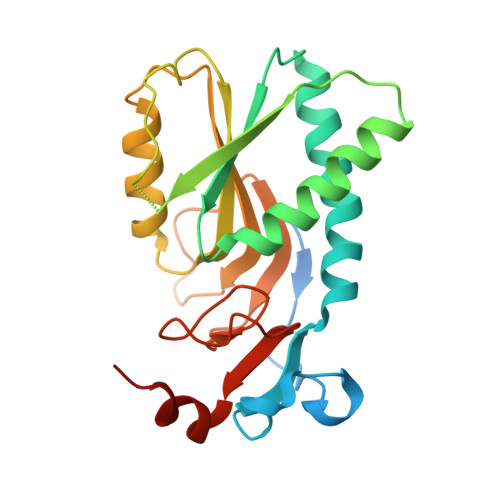
Schistosoma mansoni, the parasite responsible for schistosomiasis, lacks the "de novo" purine biosynthetic pathway and depends entirely on the purine salvage pathway for the supply of purines. Numerous reports of praziquantel resistance have been described, as well as stimulated efforts to develop new drugs against schistosomiasis. Hypoxanthine-guanine phosphoribosyltransferase (HGPRT) is a key enzyme of the purine salvage pathway. Here, we describe a crystallographic structure of the S. mansoni HPGRT-1 (SmHGPRT), complexed with IMP at a resolution of 2.8 Ǻ. Four substitutions were identified in the region of the active site between SmHGPRT-1 and human HGPRT. We also present data from RNA-Seq and WISH, suggesting that some isoforms of HGPRT might be involved in the process related to sexual maturation and reproduction in worms; furthermore, its enzymatic assays show that the isoform SmHGPRT-3 does not present the same catalytic efficiency as other isoforms. Finally, although other studies have previously suggested this enzyme as a potential antischistosomal chemotherapy target, the kinetics parameters reveal the impossibility to use SmHGPRT as an efficient chemotherapeutic target.
Organizational Affiliation:
Instituto de Física de São Carlos, Universidade de São Paulo, 13563-120, São Carlos, SP, Brazil.