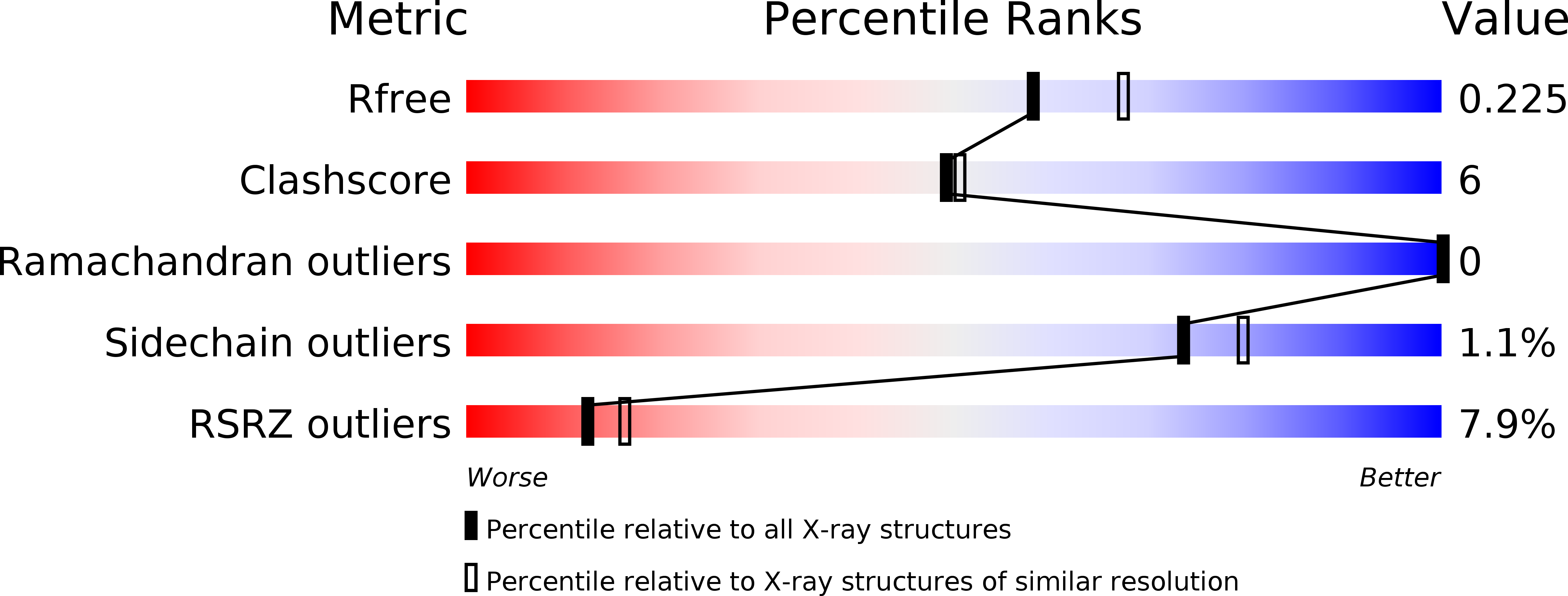
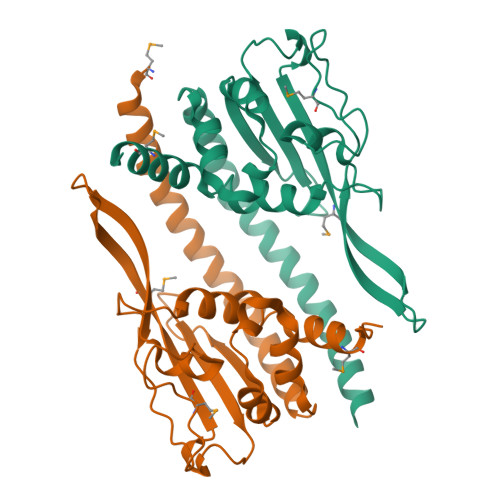
Ccp1 Homodimer Mediates Chromatin Integrity by Antagonizing CENP-A Loading
Dong, Q., Yin, F.X., Gao, F., Shen, Y., Zhang, F., Li, Y., He, H., Gonzalez, M., Yang, J., Zhang, S., Su, M., Chen, Y.H., Li, F.(2016) Mol Cell 64: 79-91
- PubMed: 27666591
- DOI: https://doi.org/10.1016/j.molcel.2016.08.022
- Primary Citation of Related Structures:
5GPK, 5GPL - PubMed Abstract:
CENP-A is a centromere-specific histone 3 variant essential for centromere specification. CENP-A partially replaces canonical histone H3 at the centromeres. How the particular CENP-A/H3 ratio at centromeres is precisely maintained is unknown. It also remains unclear how CENP-A is excluded from non-centromeric chromatin. Here, we identify Ccp1, an uncharacterized NAP family protein in fission yeast that antagonizes CENP-A loading at both centromeric and non-centromeric regions. Like the CENP-A loading factor HJURP, Ccp1 interacts with CENP-A and is recruited to centromeres at the end of mitosis in a Mis16-dependent manner. These data indicate that factors with opposing CENP-A loading activities are recruited to centromeres. Furthermore, Ccp1 also cooperates with H2A.Z to evict CENP-A assembled in euchromatin. Structural analyses indicate that Ccp1 forms a homodimer that is required for its anti-CENP-A loading activity. Our study establishes mechanisms for maintenance of CENP-A homeostasis at centromeres and the prevention of ectopic assembly of centromeres.
Organizational Affiliation:
Department of Biology, New York University, New York, NY 10003, USA.