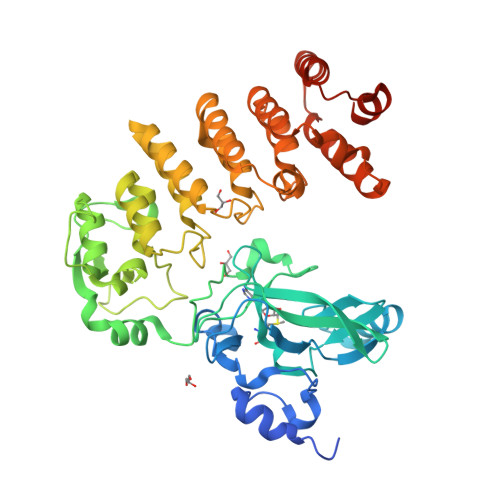
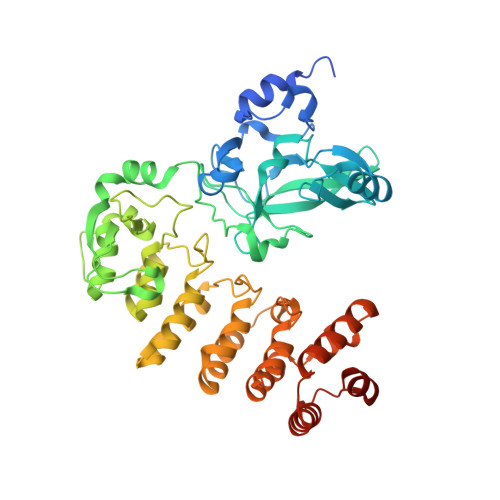
Crystal structure of Legionella pneumophila type IV secretion system effector LegAS4
Son, J., Jo, C.H., Murugan, R.N., Bang, J.K., Hwang, K.Y., Lee, W.C.(2015) Biochem Biophys Res Commun 465: 817-824
- PubMed: 26315269
- DOI: https://doi.org/10.1016/j.bbrc.2015.08.094
- Primary Citation of Related Structures:
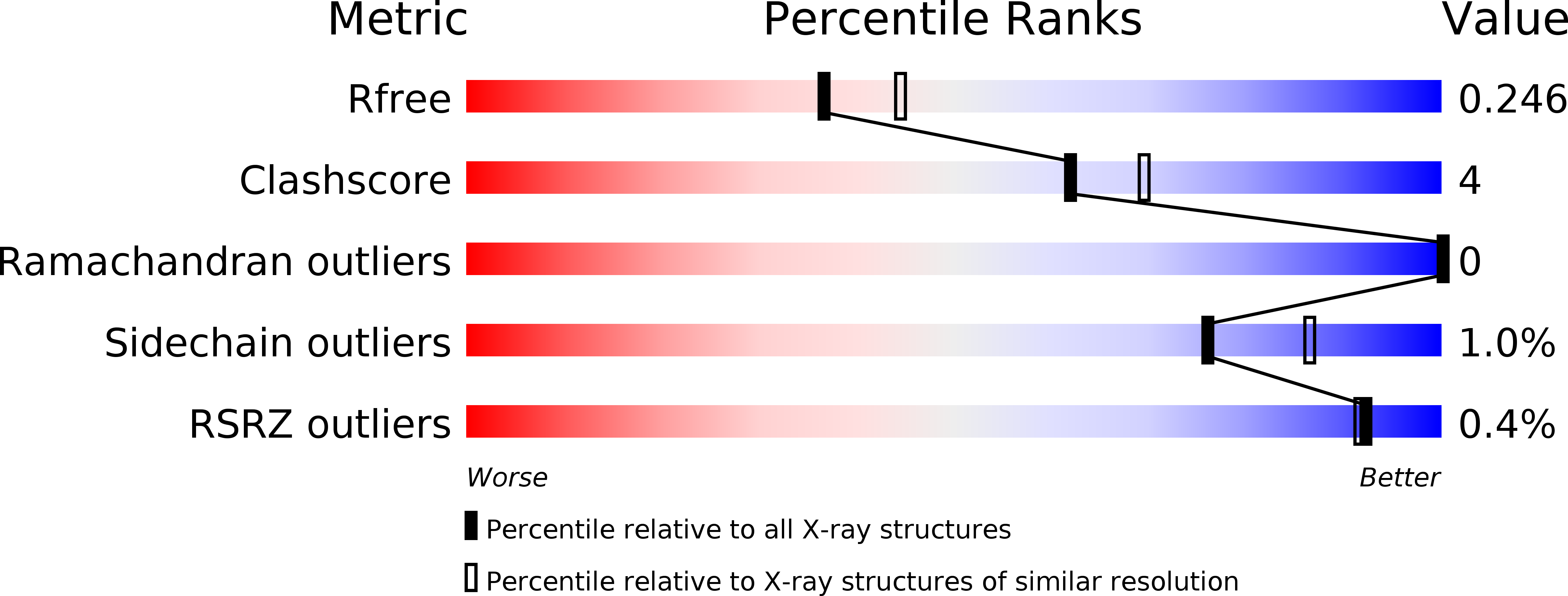
5CZY - PubMed Abstract:
The SET domain of LegAS4, a type IV secretion system effector of Legionella pneumophila, is a eukaryotic protein motif involved in histone methylation and epigenetic modulation. The SET domain of LegAS4 is involved in the modification of Lys4 of histone H3 (H3K4) in the nucleolus of the host cell, thereby enhancing heterochromatic rDNA transcription. Moreover, LegAS4 contains an ankyrin repeat domain of unknown function at its C-terminal region. Here, we report the crystal structure of LegAS4 in complex with S-adenosyl-l-methionine (SAM). Our data indicate that the ankyrin repeats interact extensively with the SET domain, especially with the SAM-binding amino acids, through conserved residues. Conserved surface analysis marks Glu159, Glu203, and Glu206 on the SET domain serve as candidate residues involved in interaction with the positively charged histone tail. Conserved surface residues on the ankyrin repeat domain surround a small pocket, which is suspected to serve as a binding site for an unknown ligand.
Organizational Affiliation:
Division of Biotechnology, Korea University, Anam-Dong, Seongbuk-Gu, Seoul 136-713, Republic of Korea.