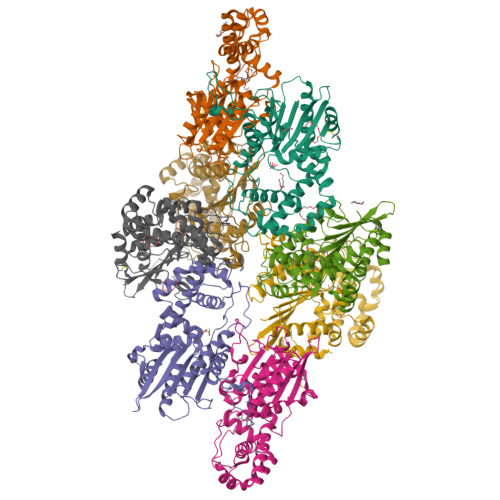
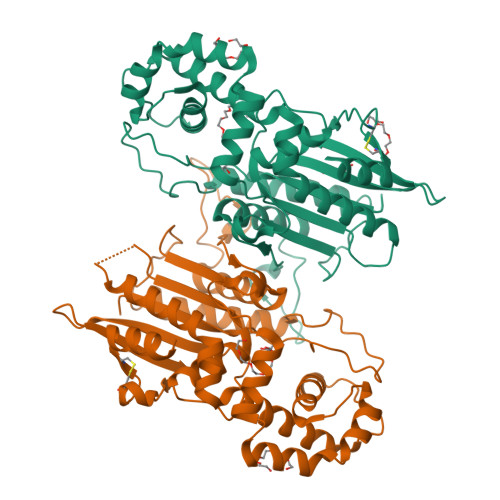
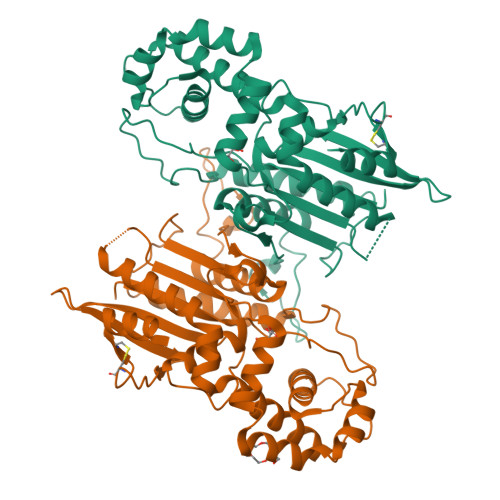
Crystal structure and tartrate inhibition of Legionella pneumophila histidine acid phosphatase.
Dhatwalia, R., Singh, H., Reilly, T.J., Tanner, J.J.(2015) Arch Biochem Biophys 585: 32-38
- PubMed: 26380880
- DOI: https://doi.org/10.1016/j.abb.2015.09.010
- Primary Citation of Related Structures:
5CDH - PubMed Abstract:
Histidine acid phosphatases (HAPs) utilize a nucleophilic histidine residue to catalyze the transfer of a phosphoryl group from phosphomonoesters to water. HAPs function as protein phosphatases and pain suppressors in mammals, are essential for Giardia lamblia excystation, and contribute to virulence of the category A pathogen Francisella tularensis. Herein we report the first crystal structure and steady-state kinetics measurements of the HAP from Legionella pneumophila (LpHAP), also known as Legionella major acid phosphatase. The structure of LpHAP complexed with the inhibitor l(+)-tartrate was determined at 2.0 Å resolution. Kinetics assays show that l(+)-tartrate is a 50-fold more potent inhibitor of LpHAP than of other HAPs. Electrostatic potential calculations provide insight into the basis for the enhanced tartrate potency: the tartrate pocket of LpHAP is more positive than other HAPs because of the absence of an ion pair partner for the second Arg of the conserved RHGXRXP HAP signature sequence. The structure also reveals that LpHAP has an atypically expansive active site entrance and lacks the nucleotide substrate base clamp found in other HAPs. These features imply that nucleoside monophosphates may not be preferred substrates. Kinetics measurements confirm that AMP is a relatively inefficient in vitro substrate of LpHAP.
Organizational Affiliation:
Department of Chemistry, University of Missouri-Columbia, Columbia, MO 65211, USA.