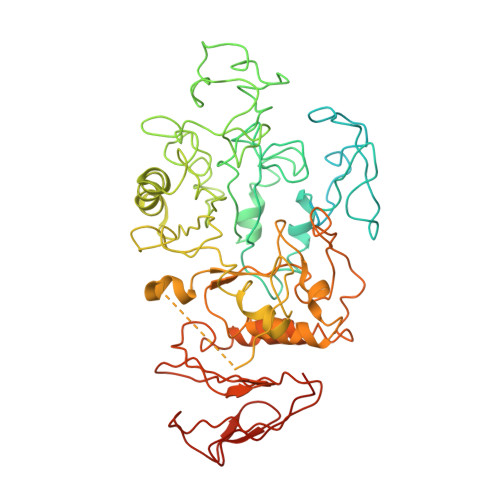
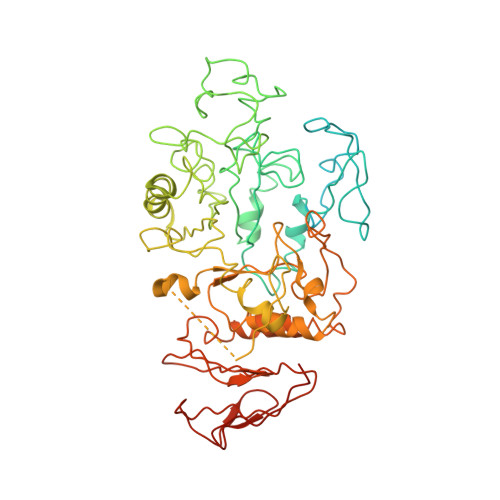
Role of N-terminal region of Escherichia coli maltodextrin glucosidase in folding and function of the protein
Pastor, A., Singh, A.K., Shukla, P.K., Equbal, M.J., Malik, S.T., Singh, T.P., Chaudhuri, T.K.(2016) Biochim Biophys Acta 1864: 1138-1151
- PubMed: 27317979
- DOI: https://doi.org/10.1016/j.bbapap.2016.06.008
- Primary Citation of Related Structures:
5BN7 - PubMed Abstract:
Maltodextrin glucosidase (MalZ) hydrolyses short malto-oligosaccharides from the reducing end releasing glucose and maltose in Escherichia coli. MalZ is a highly aggregation prone protein and molecular chaperonins GroEL and GroES assist in the folding of this protein to a substantial level. The N-terminal region of this enzyme appears to be a unique domain as seen in sequence comparison studies with other amylases as well as through homology modelling. The sequence and homology model analysis show a probability of disorder in the N-Terminal region of MalZ. The crystal structure of this enzyme has been reported in the present communication. Based on the crystallographic structure, it has been interpreted that the N-terminal region of the enzyme (Met1-Phe131) might be unstructured or flexible. To understand the role of the N-terminal region of MalZ in its enzymatic activity, and overall stability, a truncated version (Ala111-His616) of MalZ was created. The truncated version failed to fold into an active enzyme both in E. coli cytosol and in vitro even with the assistance of chaperonins GroEL and GroES. Furthermore, the refolding effort of N-truncated MalZ in the presence of isolated N-terminal domain didn't succeed. Our studies suggest that while the structural rigidity or orientation of the N-terminal region of the MalZ protein may not be essential for its stability and function, but the said domain is likely to play an important role in the formation of the native structure of the protein when present as an integral part of the protein.
Organizational Affiliation:
Kusuma School of Biological Sciences, Indian Institute of Technology Delhi, New Delhi 110016, India.