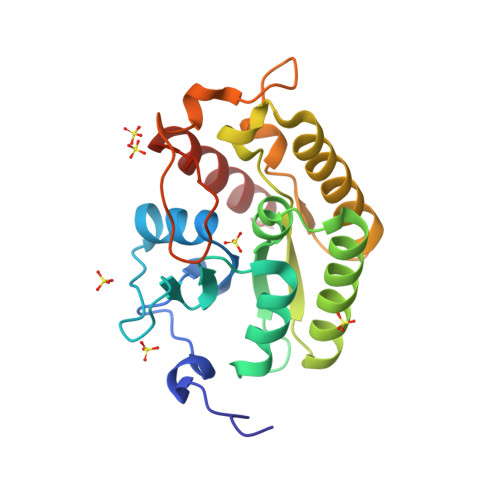
The Crystal Structure of the Drosophila Germline Inducer Oskar Identifies Two Domains with Distinct Vasa Helicase-and RNA-Binding Activities.
Jeske, M., Bordi, M., Glatt, S., Muller, S., Rybin, V., Muller, C.W., Ephrussi, A.(2015) Cell Rep 12: 587
- PubMed: 26190108
- DOI: https://doi.org/10.1016/j.celrep.2015.06.055
- Primary Citation of Related Structures:
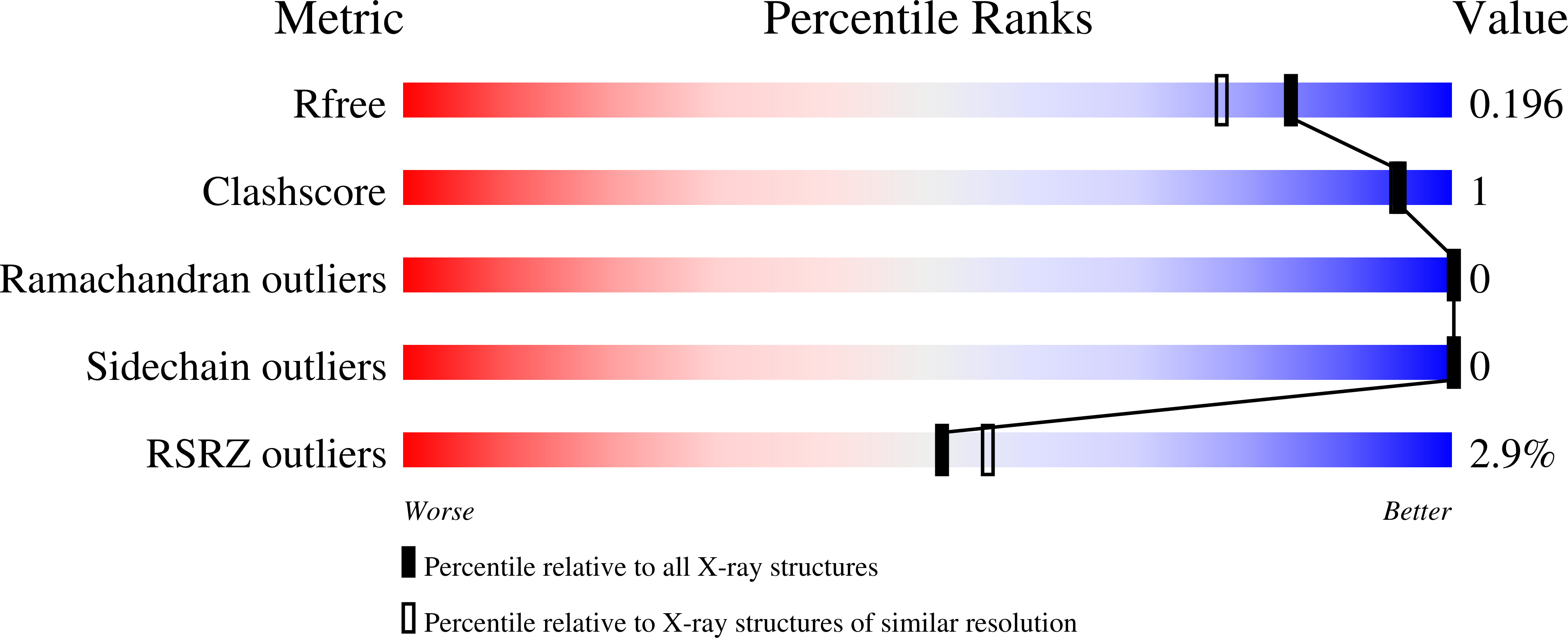
5A48, 5A49, 5A4A - PubMed Abstract:
In many animals, the germ plasm segregates germline from soma during early development. Oskar protein is known for its ability to induce germ plasm formation and germ cells in Drosophila. However, the molecular basis of germ plasm formation remains unclear. Here, we show that Oskar is an RNA-binding protein in vivo, crosslinking to nanos, polar granule component, and germ cell-less mRNAs, each of which has a role in germline formation. Furthermore, we present high-resolution crystal structures of the two Oskar domains. RNA-binding maps in vitro to the C-terminal domain, which shows structural similarity to SGNH hydrolases. The highly conserved N-terminal LOTUS domain forms dimers and mediates Oskar interaction with the germline-specific RNA helicase Vasa in vitro. Our findings suggest a dual function of Oskar in RNA and Vasa binding, providing molecular clues to its germ plasm function.
Organizational Affiliation:
Developmental Biology Unit, European Molecular Biology Laboratory, 69117 Heidelberg, Germany; Structural and Computational Biology Unit, European Molecular Biology Laboratory, 69117 Heidelberg, Germany.