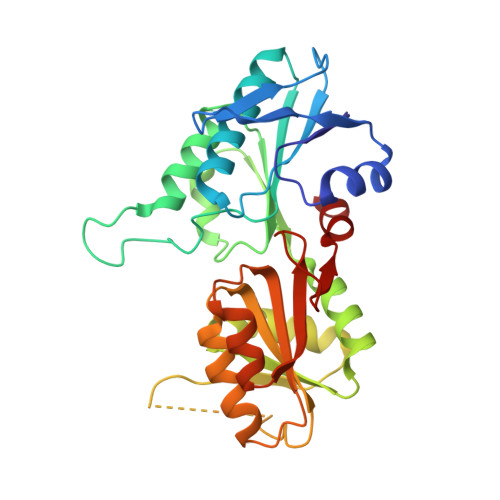
Structure of dimeric, recombinant Sulfolobus solfataricus phosphoribosyl diphosphate synthase: a bent dimer defining the adenine specificity of the substrate ATP.
Andersen, R.W., Leggio, L.L., Hove-Jensen, B., Kadziola, A.(2015) Extremophiles 19: 407-415
- PubMed: 25605536
- DOI: https://doi.org/10.1007/s00792-014-0726-x
- Primary Citation of Related Structures:
4TWB - PubMed Abstract:
The enzyme 5-phosphoribosyl-1-α-diphosphate (PRPP) synthase (EC 2.7.6.1) catalyses the Mg(2+)-dependent transfer of a diphosphoryl group from ATP to the C1 hydroxyl group of ribose 5-phosphate resulting in the production of PRPP and AMP. A nucleotide sequence specifying Sulfolobus solfataricus PRPP synthase was synthesised in vitro with optimised codon usage for expression in Escherichia coli. Following expression of the gene in E. coli PRPP synthase was purified by heat treatment and ammonium sulphate precipitation and the structure of S. solfataricus PRPP synthase was determined at 2.8 Å resolution. A bent dimer oligomerisation was revealed, which seems to be an abundant feature among PRPP synthases for defining the adenine specificity of the substrate ATP. Molecular replacement was used to determine the S. solfataricus PRPP synthase structure with a monomer subunit of Methanocaldococcus jannaschii PRPP synthase as a search model. The two amino acid sequences share 35 % identity. The resulting asymmetric unit consists of three separated dimers. The protein was co-crystallised in the presence of AMP and ribose 5-phosphate, but in the electron density map of the active site only AMP and a sulphate ion were observed. Sulphate ion, reminiscent of the ammonium sulphate precipitation step of the purification, seems to bind tightly and, therefore, presumably occupies and blocks the ribose 5-phosphate binding site. The activity of S. solfataricus PRPP synthase is independent of phosphate ion.
Organizational Affiliation:
Department of Chemistry, University of Copenhagen, 5 Universitetsparken, 2100, Copenhagen Ø, Denmark.