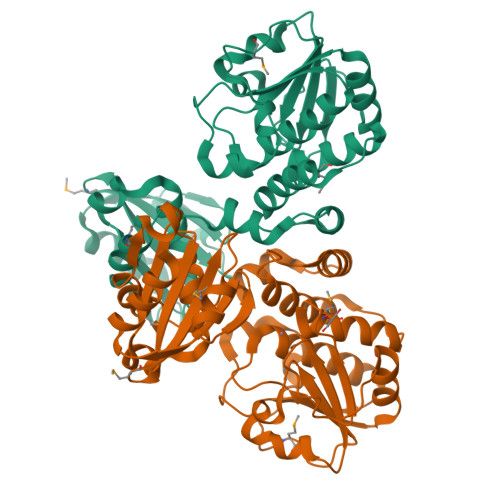
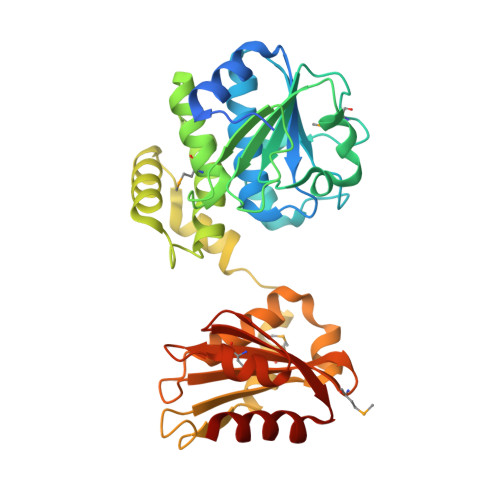
Unique subunit packing in mycobacterial nanoRNase leads to alternate substrate recognitions in DHH phosphodiesterases
Srivastav, R., Kumar, D., Grover, A., Singh, A., Manjasetty, B.A., Sharma, R., Taneja, B.(2014) Nucleic Acids Res 42: 7894-7910
- PubMed: 24878921
- DOI: https://doi.org/10.1093/nar/gku425
- Primary Citation of Related Structures:
4LS9 - PubMed Abstract:
DHH superfamily includes RecJ, nanoRNases (NrnA), cyclic nucleotide phosphodiesterases and pyrophosphatases. In this study, we have carried out in vitro and in vivo investigations on the bifunctional NrnA-homolog from Mycobacterium smegmatis, MSMEG_2630. The crystal structure of MSMEG_2630 was determined to 2.2-Å resolution and reveals a dimer consisting of two identical subunits with each subunit folding into an N-terminal DHH domain and a C-terminal DHHA1 domain. The overall structure and fold of the individual domains is similar to other members of DHH superfamily. However, MSMEG_2630 exhibits a distinct quaternary structure in contrast to other DHH phosphodiesterases. This novel mode of subunit packing and variations in the linker region that enlarge the domain interface are responsible for alternate recognitions of substrates in the bifunctional nanoRNases. MSMEG_2630 exhibits bifunctional 3'-5' exonuclease [on both deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) substrates] as well as CysQ-like phosphatase activity (on pAp) in vitro with a preference for nanoRNA substrates over single-stranded DNA of equivalent lengths. A transposon disruption of MSMEG_2630 in M. smegmatis causes growth impairment in the presence of various DNA-damaging agents. Further phylogenetic analysis and genome organization reveals clustering of bacterial nanoRNases into two distinct subfamilies with possible role in transcriptional and translational events during stress.
Organizational Affiliation:
CSIR-IGIB, Institute of Genomics and Integrative Biology, South Campus Mathura Road, New Delhi 110020, India.