A Novel Allosteric Inhibitor of the Uridine Diphosphate N-Acetylglucosamine Pyrophosphorylase from Trypanosoma Brucei.
Urbaniak, M.D., Collie, I.T., Fang, W., Aristotelous, T., Eskilsson, S., Raimi, O.G., Harrison, J., Hopkins Navratolova, I., Frearson, J.A., Van Aalten, D.M.F., Ferguson, M.A.J.(2013) ACS Chem Biol 8: 1981
- PubMed: 23834437
- DOI: https://doi.org/10.1021/cb400411x
- Primary Citation of Related Structures:
4BQH - PubMed Abstract:
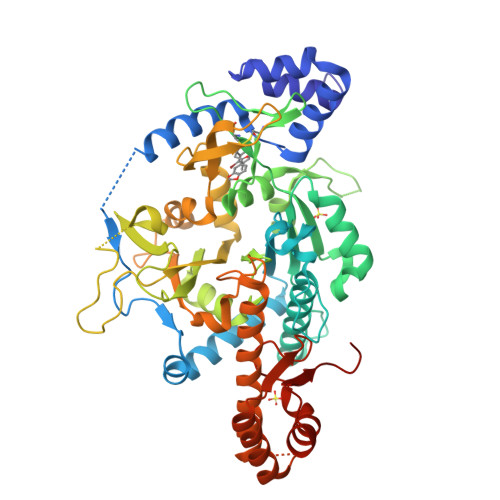
Uridine diphosphate N-acetylglucosamine pyrophosphorylase (UAP) catalyzes the final reaction in the biosynthesis of UDP-GlcNAc, an essential metabolite in many organisms including Trypanosoma brucei, the etiological agent of Human African Trypanosomiasis. High-throughput screening of recombinant T. brucei UAP identified a UTP-competitive inhibitor with selectivity over the human counterpart despite the high level of conservation of active site residues. Biophysical characterization of the UAP enzyme kinetics revealed that the human and trypanosome enzymes both display a strictly ordered bi-bi mechanism, but with the order of substrate binding reversed. Structural characterization of the T. brucei UAP-inhibitor complex revealed that the inhibitor binds at an allosteric site absent in the human homologue that prevents the conformational rearrangement required to bind UTP. The identification of a selective inhibitory allosteric binding site in the parasite enzyme has therapeutic potential.
Organizational Affiliation:
Division of Biological Chemistry and Drug Discovery, ‡Division of Molecular Microbiology, and §MRC Protein Phosphorylation and Ubiquitylation Unit, College of Life Sciences, University of Dundee , Dundee DD1 5EH, U.K.