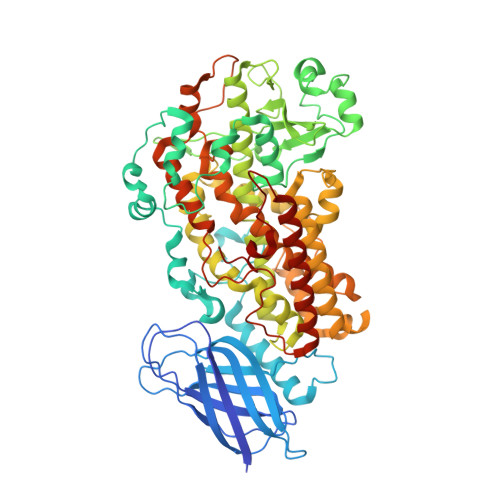
Structure of a Calcium-dependent 11R-Lipoxygenase Suggests a Mechanism for Ca2+ Regulation.
Eek, P., Jarving, R., Jarving, I., Gilbert, N.C., Newcomer, M.E., Samel, N.(2012) J Biol Chem 287: 22377-22386
- PubMed: 22573333
- DOI: https://doi.org/10.1074/jbc.M112.343285
- Primary Citation of Related Structures:
3VF1 - PubMed Abstract:
Lipoxygenases (LOXs) are a key part of several signaling pathways that lead to inflammation and cancer. Yet, the mechanisms of substrate binding and allosteric regulation by the various LOX isoforms remain speculative. Here we report the 2.47-Å resolution crystal structure of the arachidonate 11R-LOX from Gersemia fruticosa, which sheds new light on the mechanism of LOX catalysis. Our crystallographic and mutational studies suggest that the aliphatic tail of the fatty acid is bound in a hydrophobic pocket with two potential entrances. We speculate that LOXs share a common T-shaped substrate channel architecture that gives rise to the varying positional specificities. A general allosteric mechanism is proposed for transmitting the activity-inducing effect of calcium binding from the membrane-targeting PLAT (polycystin-1/lipoxygenase/α-toxin) domain to the active site via a conserved π-cation bridge.
Organizational Affiliation:
Department of Chemistry, Tallinn University of Technology, Akadeemia tee 15, 12618 Tallinn, Estonia.