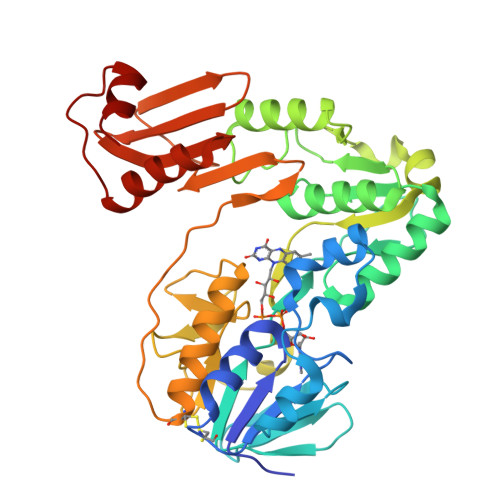
Crystal structure of NADH:rubredoxin oxidoreductase from Clostridium acetobutylicum: a key component of the dioxygen scavenging system in obligatory anaerobes.
Nishikawa, K., Shomura, Y., Kawasaki, S., Niimura, Y., Higuchi, Y.(2010) Proteins 78: 1066-1070
- PubMed: 20017214
- DOI: https://doi.org/10.1002/prot.22650
- Primary Citation of Related Structures:
3KLJ
Organizational Affiliation:
Department of Life Science, Graduate School of Life Science, University of Hyogo, 3-2-1 Koto, Kamigori-cho, Ako-gun, Hyogo 678-1297, Japan.