Crystal Structure of Protein Ph0793 from Pyrococcus Horikoshii
Chang, C., Skarina, T., Savchenko, A., Edwards, A., Joachimiak, A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| putative transferase PH0793 | 278 | Pyrococcus horikoshii | Mutation(s): 0 Gene Names: PH0793 EC: 2.5.1.114 | 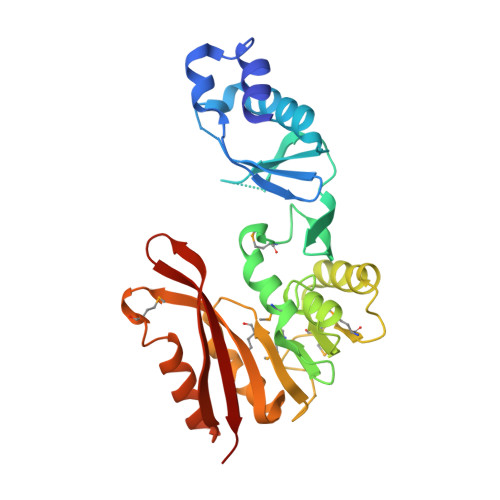 | |
UniProt | |||||
Find proteins for O58523 (Pyrococcus horikoshii (strain ATCC 700860 / DSM 12428 / JCM 9974 / NBRC 100139 / OT-3)) Explore O58523 Go to UniProtKB: O58523 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O58523 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 51.308 | α = 90 |
| b = 54.231 | β = 100.02 |
| c = 58.613 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SBC-Collect | data collection |
| HKL-3000 | phasing |
| SHELXE | model building |
| SOLVE | phasing |
| RESOLVE | model building |
| ARP/wARP | model building |
| REFMAC | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| RESOLVE | phasing |