Structural basis of an ERAD pathway mediated by the ER-resident protein disulfide reductase ERdj5.
Hagiwara, M., Maegawa, K., Suzuki, M., Ushioda, R., Araki, K., Matsumoto, Y., Hoseki, J., Nagata, K., Inaba, K.(2011) Mol Cell 41: 432-444
- PubMed: 21329881
- DOI: https://doi.org/10.1016/j.molcel.2011.01.021
- Primary Citation of Related Structures:
3APO, 3APQ, 3APS - PubMed Abstract:
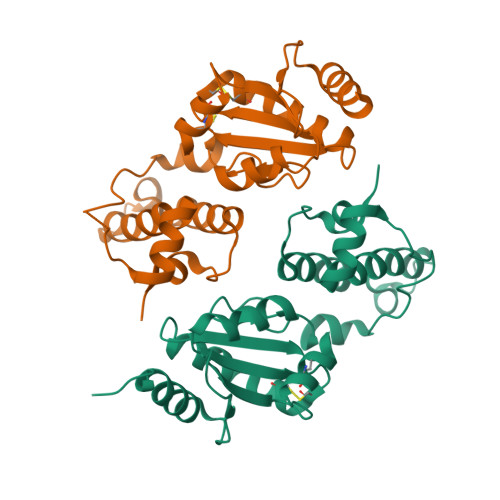
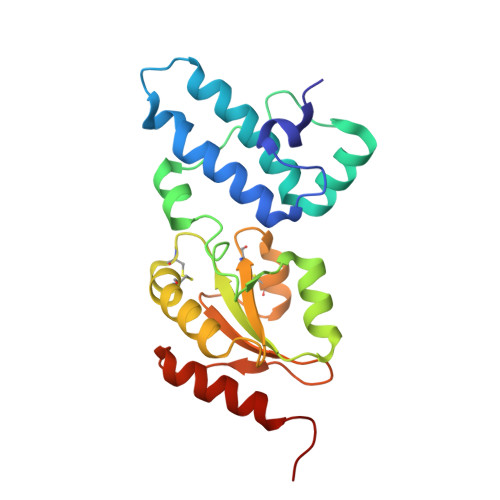
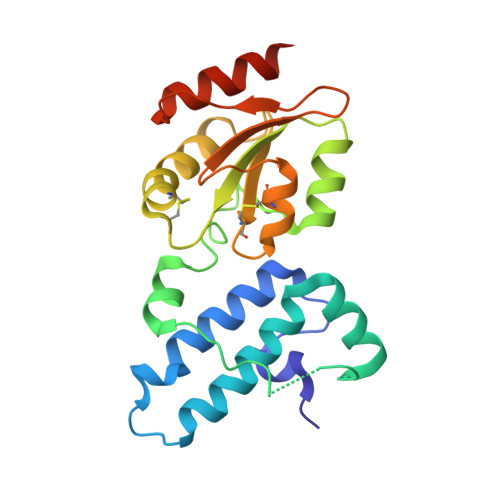
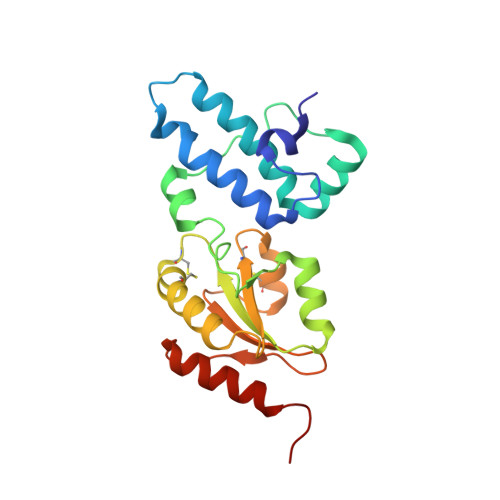
ER-associated degradation (ERAD) is an ER quality-control process that eliminates terminally misfolded proteins. ERdj5 was recently discovered to be a key ER-resident PDI family member protein that accelerates ERAD by reducing incorrect disulfide bonds in misfolded glycoproteins recognized by EDEM1. We here solved the crystal structure of full-length ERdj5, thereby revealing that ERdj5 contains the N-terminal J domain and six tandem thioredoxin domains that can be divided into the N- and C-terminal clusters. Our systematic biochemical analyses indicated that two thioredoxin domains that constitute the C-terminal cluster form the highly reducing platform that interacts with EDEM1 and reduces EDEM1-recruited substrates, leading to their facilitated degradation. The pulse-chase experiment further provided direct evidence for the sequential movement of an ERAD substrate from calnexin to the downstream EDEM1-ERdj5 complex, and then to the retrotranslocation channel, probably through BiP. We present a detailed molecular view of how ERdj5 mediates ERAD in concert with EDEM1.
Organizational Affiliation:
Department of Molecular and Cellular Biology, Institute for Frontier Medical Sciences, Kyoto University, Kyoto 606-8507, Japan.