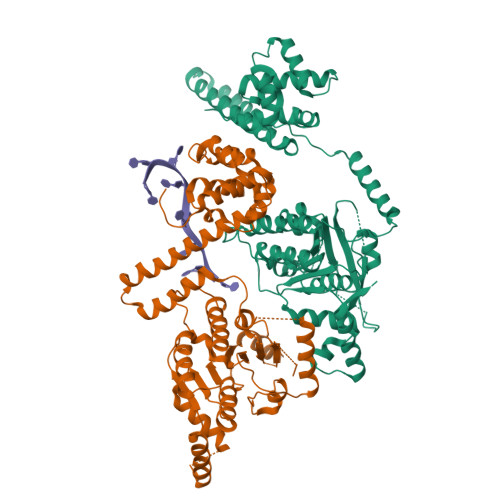
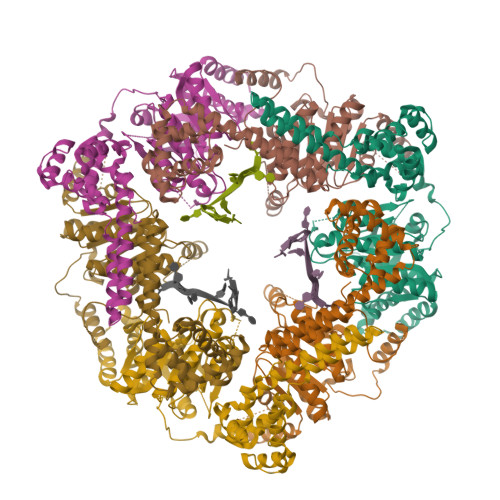
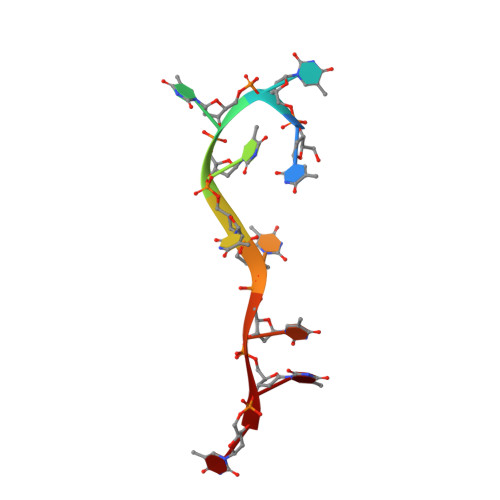
The Crystal Structure of a Replicative Hexameric Helicase Dnac and its Complex with Single-Stranded DNA.
Lo, Y.H., Tsai, K.L., Sun, Y.J., Chen, W.T., Huang, C.Y., Hsiao, C.D.(2009) Nucleic Acids Res 37: 804
- PubMed: 19074952
- DOI: https://doi.org/10.1093/nar/gkn999
- Primary Citation of Related Structures:
2VYE, 2VYF - PubMed Abstract:
DNA helicases are motor proteins that play essential roles in DNA replication, repair and recombination. In the replicative hexameric helicase, the fundamental reaction is the unwinding of duplex DNA; however, our understanding of this function remains vague due to insufficient structural information. Here, we report two crystal structures of the DnaB-family replicative helicase from Geobacillus kaustophilus HTA426 (GkDnaC) in the apo-form and bound to single-stranded DNA (ssDNA). The GkDnaC-ssDNA complex structure reveals that three symmetrical basic grooves on the interior surface of the hexamer individually encircle ssDNA. The ssDNA-binding pockets in this structure are directed toward the N-terminal domain collar of the hexameric ring, thus orienting the ssDNA toward the DnaG primase to facilitate the synthesis of short RNA primers. These findings provide insight into the mechanism of ssDNA binding and provide a working model to establish a novel mechanism for DNA translocation at the replication fork.
Organizational Affiliation:
Institute of Molecular Biology, Academia Sinica, Taipei, 115, Taiwan.