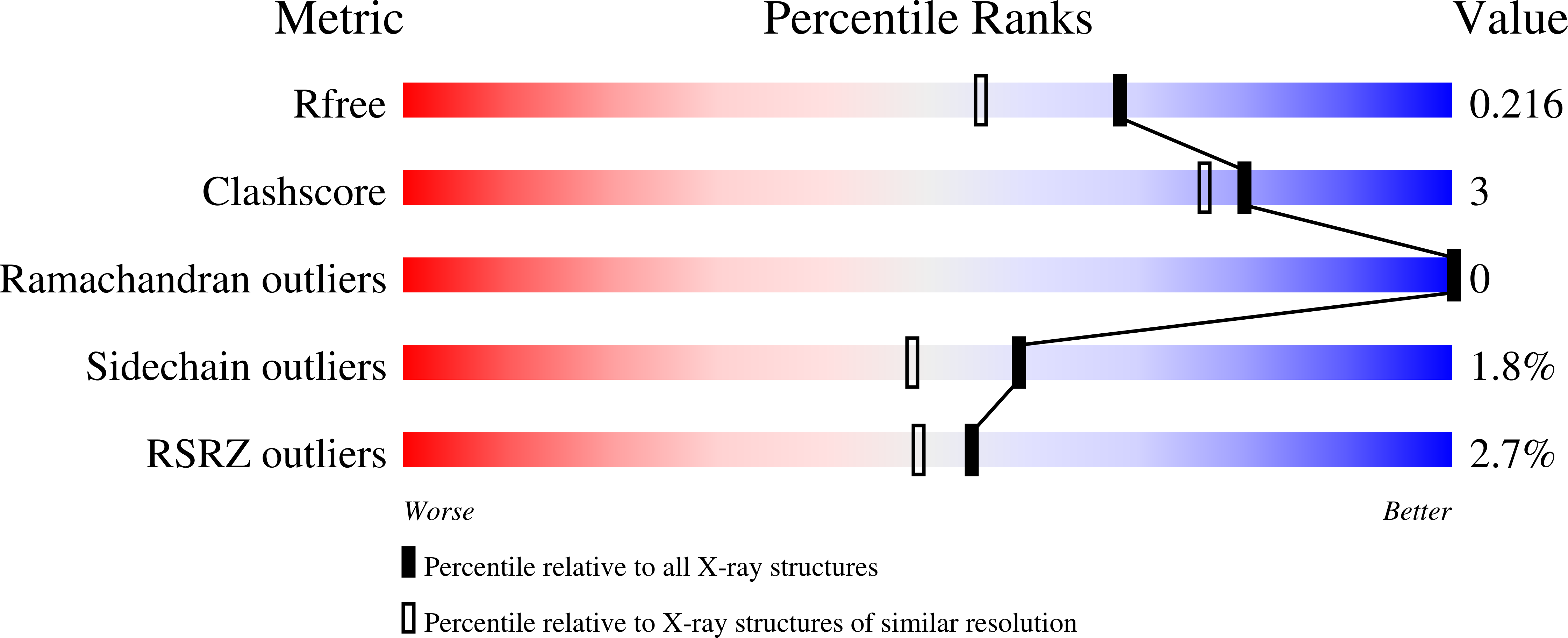
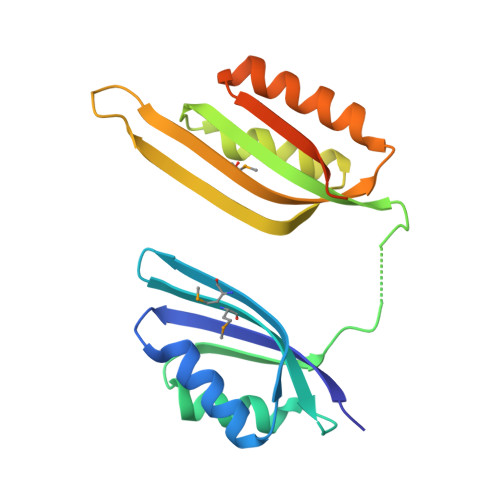
Crystal structure of tandem ACT domain-containing protein ACTP from Galdieria sulphuraria.
Bitto, E., Kim, D.J., Bingman, C.A., Kim, H.J., Han, B.W., Phillips, G.N.(2012) Proteins 80: 2105-2109
- PubMed: 22528523
- DOI: https://doi.org/10.1002/prot.24101
- Primary Citation of Related Structures:
2NYI - PubMed Abstract:
The ACT domain is a structurally conserved small molecule binding domain which is mostly involved in amino acid and purine metabolism. Here, we report the crystal structure of a tandem ACT domain-containing protein (ACTP) from Galdieria sulphuraria. The two ACTP monomers in the asymmetric unit form a dimer with a non-crystallographic twofold axis in a domain-swapped manner, showing a horseshoe-like structure with a central crevice. This structure contributes to expand our knowledge on the structural diversity of ACT domain-containing proteins.
Organizational Affiliation:
Department of Chemistry and Biochemistry, Georgian Court University, Lakewood, New Jersey 08701, USA.