Structure of D-ribulose 5-phosphate 3-epimerase from Synechocystis to 1.6 A resolution.
Wise, E.L., Akana, J., Gerlt, J.A., Rayment, I.(2004) Acta Crystallogr D Biol Crystallogr 60: 1687-1690
- PubMed: 15333955
- DOI: https://doi.org/10.1107/S0907444904015896
- Primary Citation of Related Structures:
1TQJ - PubMed Abstract:
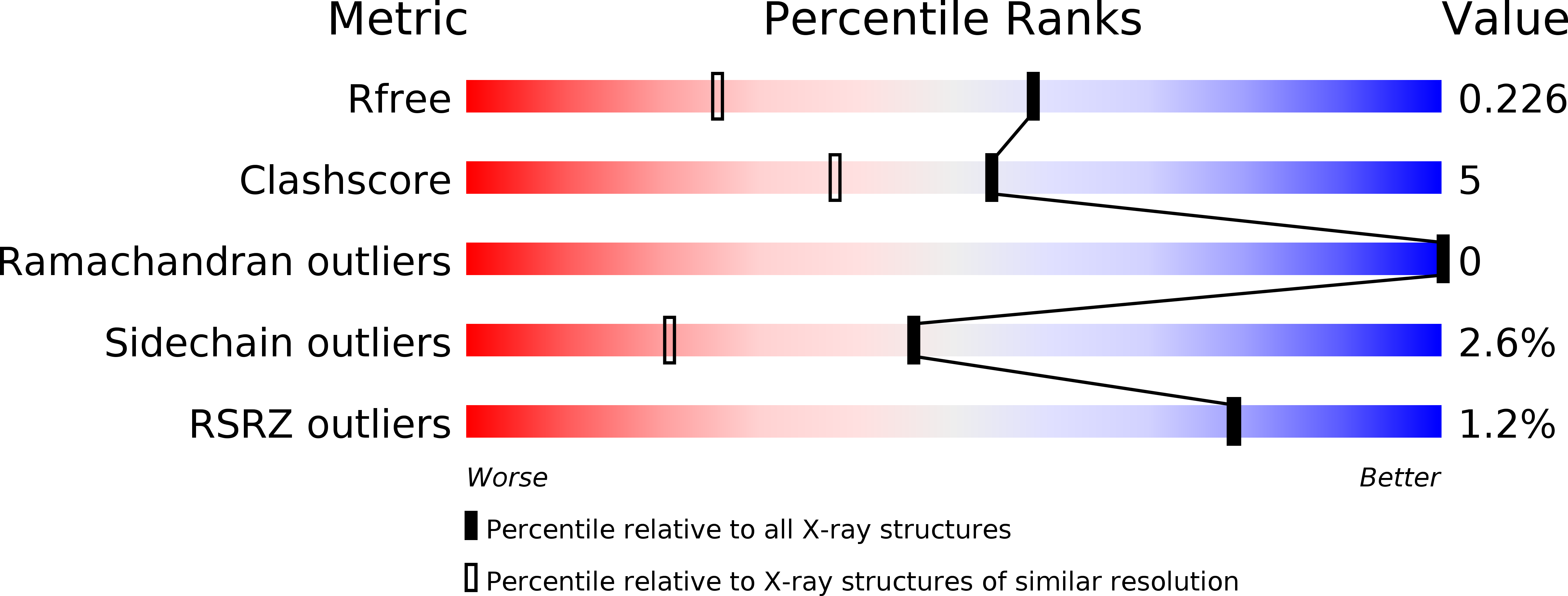
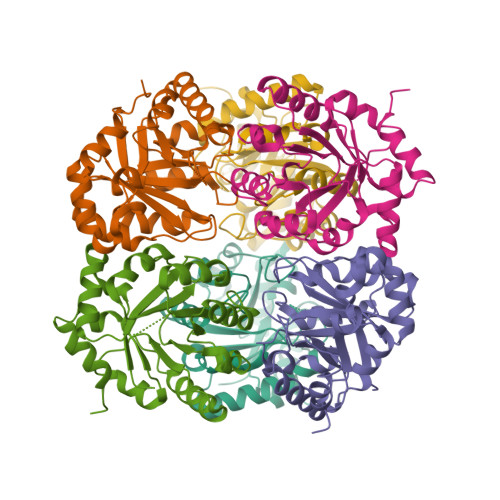
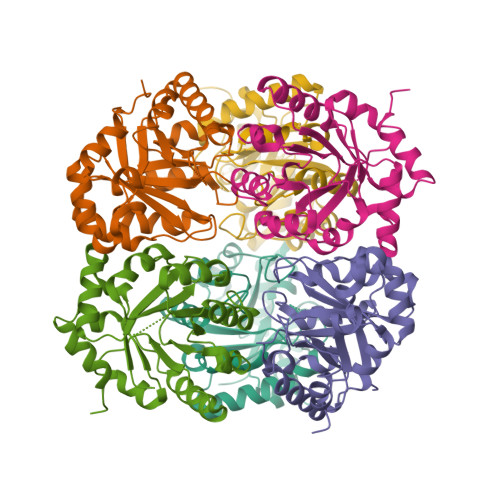
The crystal structure of D-ribulose 5-phosphate 3-epimerase (RPE) from the cyanobacterium Synechocystis was determined by X-ray crystallography to 1.6 A resolution. The enzyme, which catalyzes the epimerization of D-ribulose 5-phosphate and D-xylulose 5-phosphate, assembles as a hexamer of (beta/alpha)(8)-barrels in the crystallographic asymmetric unit. The active site is highly similar to those of two previously reported RPEs and provides further evidence for essential catalytic roles for several active-site residues.
Organizational Affiliation:
Deparment of Biochemistry, University of Wisconsin, Madison, WI 53706, USA.