Unusual space-group pseudosymmetry in crystals of human phosphopantothenoylcysteine decarboxylase.
Manoj, N., Ealick, S.E.(2003) Acta Crystallogr D Biol Crystallogr 59: 1762-1766
- PubMed: 14501115
- DOI: https://doi.org/10.1107/s0907444903016214
- Primary Citation of Related Structures:
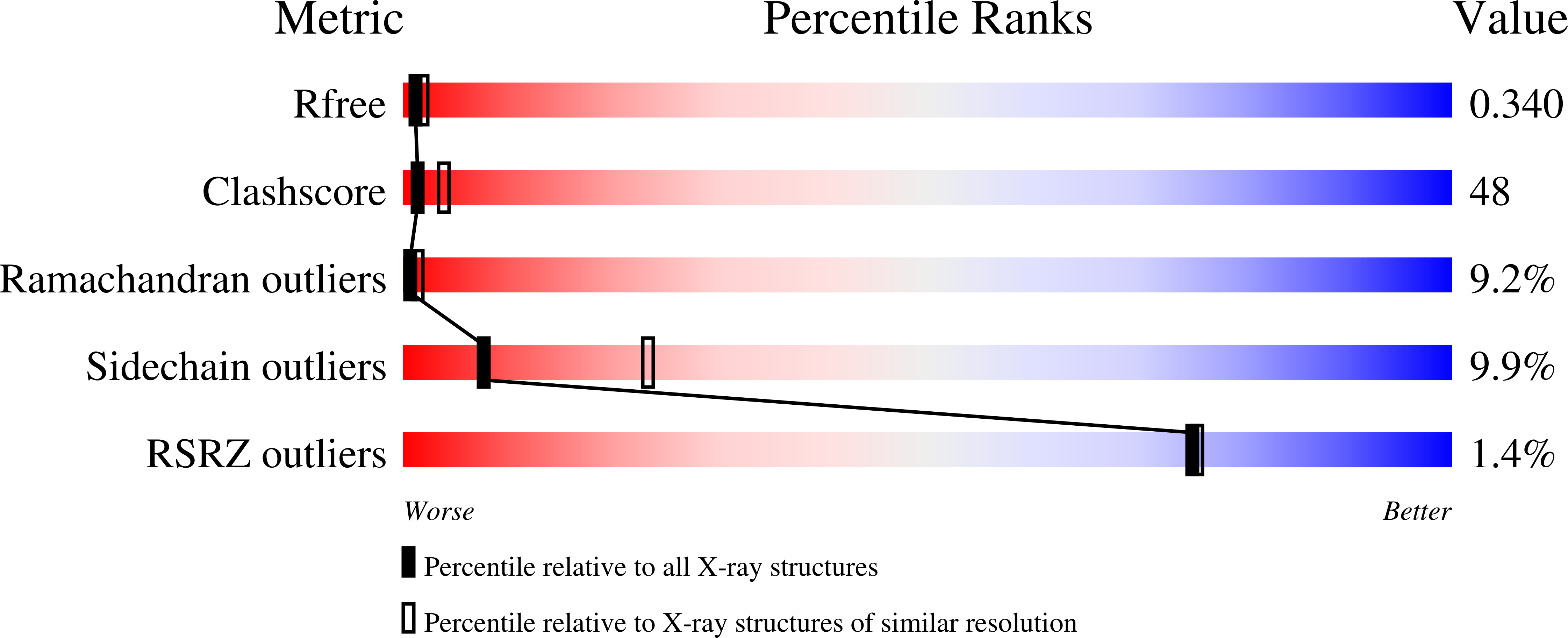
1QZU - PubMed Abstract:
Phosphopantothenoylcysteine (PPC) decarboxylase is an essential enzyme in the biosynthesis of coenzyme A and catalyzes the decarboxylation of PPC to phosphopantetheine. Human PPC decarboxylase has been expressed in Escherichia coli, purified and crystallized. The Laue class of the diffraction data appears to be 3m, suggesting space group R32 with two monomers per asymmetric unit. However, the crystals belong to the space group R3 and the asymmetric unit contains four monomers. The structure has been solved using molecular replacement and refined to a current R factor of 29%. The crystal packing can be considered as two interlaced lattices, each consistent with space group R32 and with the corresponding twofold axes parallel to each other but separated along the threefold axis. Thus, the true space group is R3 with four monomers per asymmetric unit.
Organizational Affiliation:
Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853, USA.