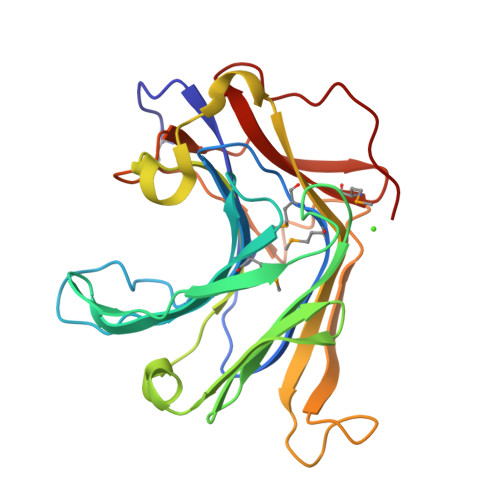
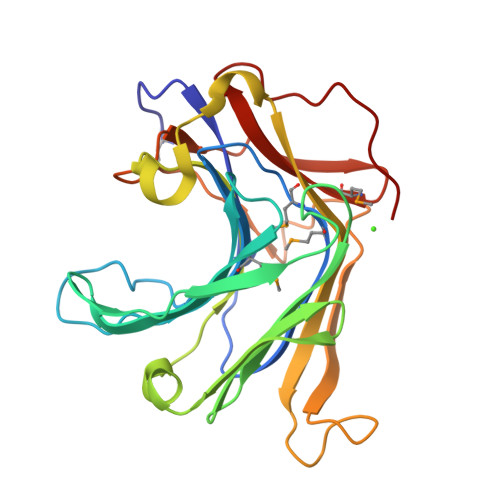
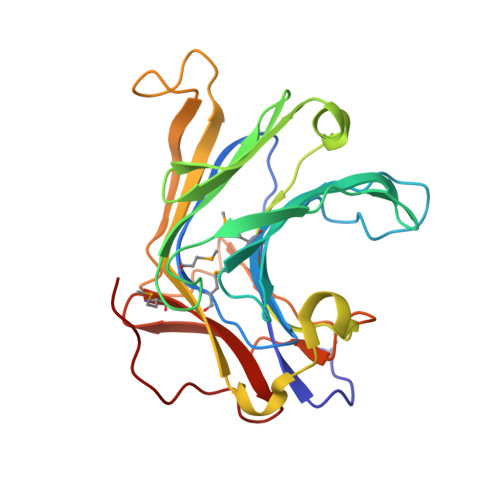
Crystal Structure of a Natural Circularly Permuted Jellyroll Protein: 1,3-1,4-beta-D-Glucanase from Fibrobacter succinogenes.
Tsai, L.C., Shyur, L.F., Lee, S.H., Lin, S.S., Yuan, H.S.(2003) J Mol Biology 330: 607-620
- PubMed: 12842475
- DOI: https://doi.org/10.1016/s0022-2836(03)00630-2
- Primary Citation of Related Structures:
1MVE - PubMed Abstract:
The 1,3-1,4-beta-D-glucanase from Fibrobacter succinogenes (Fsbeta-glucanase) is classified as one of the family 16 glycosyl hydrolases. It hydrolyzes the glycosidic bond in the mixed-linked glucans containing beta-1,3- and beta-1,4-glycosidic linkages. We constructed a truncated form of recombinant Fsbeta-glucanase containing the catalytic domain from amino acid residues 1-258, which exhibited a higher thermal stability and enzymatic activity than the full-length enzyme. The crystal structure of the truncated Fsbeta-glucanase was solved at a resolution of 1.7A by the multiple wavelength anomalous dispersion (MAD) method using the anomalous signals from the seleno-methionine-labeled protein. The overall topology of the truncated Fsbeta-glucanase consists mainly of two eight-stranded anti-parallel beta-sheets arranged in a jellyroll beta-sandwich, similar to the fold of many glycosyl hydrolases and carbohydrate-binding modules. Sequence comparison with other bacterial glucanases showed that Fsbeta-glucanase is the only naturally occurring circularly permuted beta-glucanase with reversed sequences. Structural comparison shows that the engineered circular-permuted Bacillus enzymes are more similar to their parent enzymes with which they share approximately 70% sequence identity, than to the naturally occurring Fsbeta-glucanase of similar topology with 30% identity. This result suggests that protein structure relies more on sequence identity than topology. The high-resolution structure of Fsbeta-glucanase provides a structural rationale for the different activities obtained from a series of mutant glucanases and a basis for the development of engineered enzymes with increased activity and structural stability.
Organizational Affiliation:
Institute of Molecular Biology, Academia Sinica, Taipei, Taiwan, ROC.