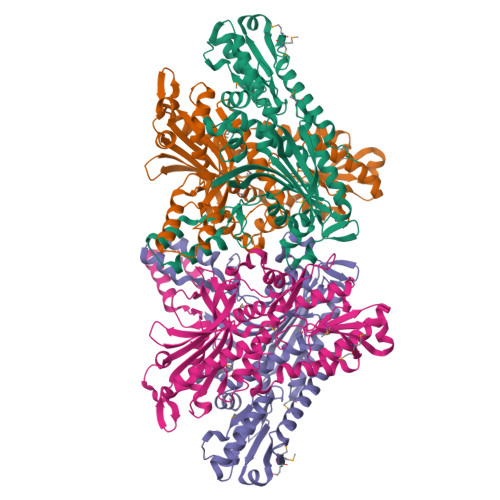
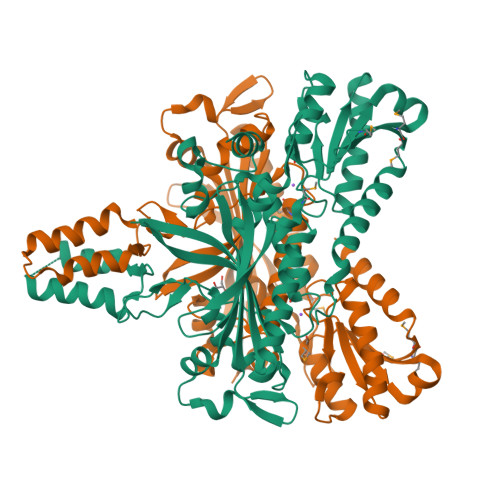
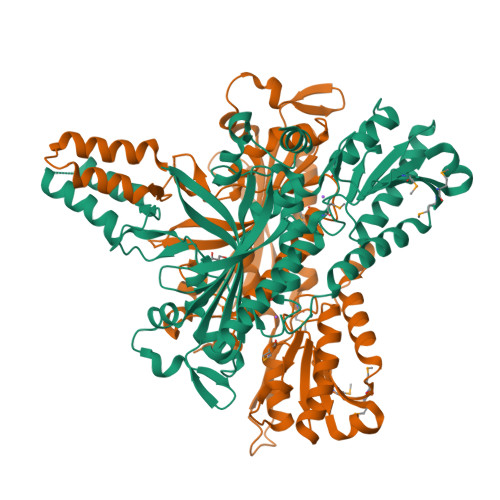
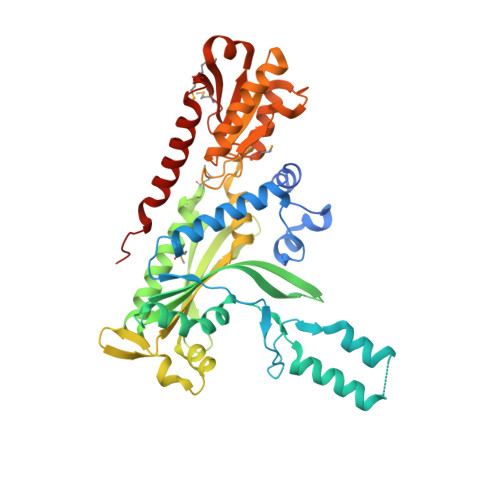
Crystal structure and deletion analysis show that the accessory subunit of mammalian DNA polymerase gamma, Pol gamma B, functions as a homodimer.
Carrodeguas, J.A., Theis, K., Bogenhagen, D.F., Kisker, C.(2001) Mol Cell 7: 43-54
- PubMed: 11172710
- DOI: https://doi.org/10.1016/s1097-2765(01)00153-8
- Primary Citation of Related Structures:
1G5H, 1G5I - PubMed Abstract:
Polymerase gamma, which replicates and repairs mitochondrial DNA, requires the Pol gamma B subunit for processivity. We determined the crystal structure of mouse Pol gamma B, a core component of the mitochondrial replication machinery. Pol gamma B shows high similarity to glycyl-tRNA synthetase and dimerizes through an unusual intermolecular four-helix bundle. A human Pol gamma B mutant lacking the four-helix bundle failed to dimerize in solution or to stimulate the catalytic subunit Pol gamma A, but retained the ability to bind with Pol gamma A to a primer-template construct, indicating that the functional holoenzyme contains two Pol gamma B molecules. Other mutants retained stimulatory activity but lost the ability to bind folded ssDNA. These results suggest that the Pol gamma B dimer contains distinct sites for Pol gamma A binding, dimerization, and DNA binding.
Organizational Affiliation:
Department of Pharmacological Sciences, State University of New York at Stony Brook, Stony Brook, NY 11794, USA.