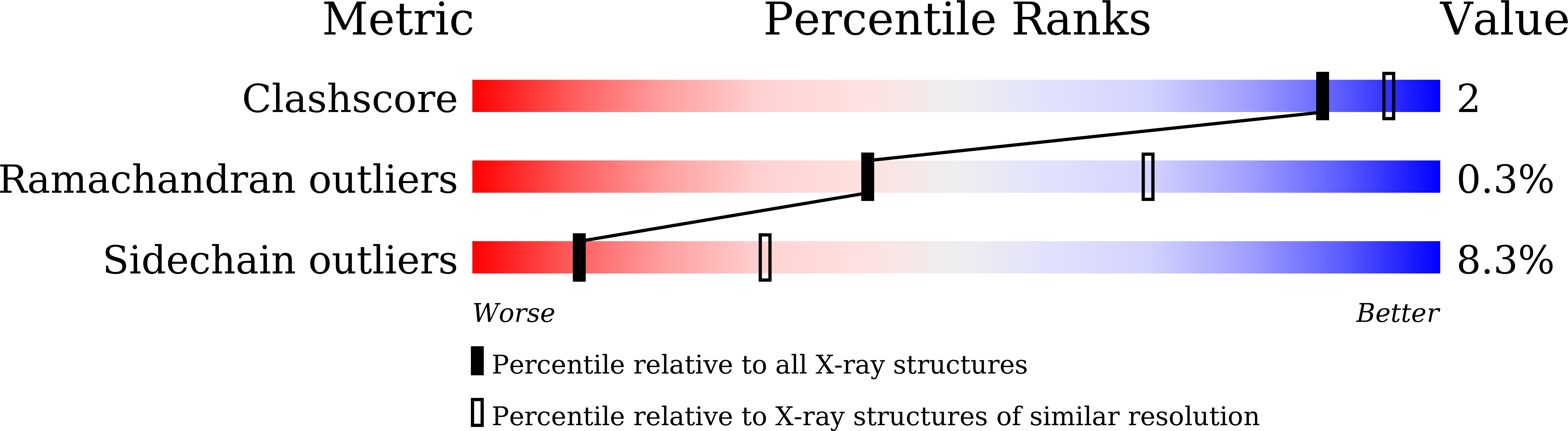Discovery of Novel Phenoxyaryl Pyridones as Bromodomain and Extra-Terminal Domain (BET) Inhibitors with High Selectivity for the Second Bromodomain (BD2) to Potentially Treat Acute Myeloid Leukemia.
Jiang, W., Hou, Q., Xu, H., Yang, K., Wang, X., Zhang, K., Zeng, Y., Li, W., Wang, B., Luo, G., Zhao, X., Shen, H., Xu, Y., Wu, X.(2024) J Med Chem 67: 1513-1532
- PubMed: 38175809
- DOI: https://doi.org/10.1021/acs.jmedchem.3c02104
- Primary Citation of Related Structures:
8WXY, 8WY3, 8WY7, 8WYG - PubMed Abstract:
Bromodomain-selective BET inhibition has emerged as a promising strategy to improve the safety profiles of pan -BET inhibitors. Herein, we report the discovery of potent phenoxyaryl pyridones as highly BD2-selective BET inhibitors. Compound 23 (IC 50 = 2.9 nM) exhibited a comparable BRD4 BD2 inhibitory activity relative to 10 (IC 50 = 1.0 nM) and remarkably improved selectivity over BRD4 BD1 ( 23 : 2583-fold; 10 : 344-fold). This lead compound significantly inhibited the proliferation of acute myeloid leukemia (AML) cell lines through induction of G0/G1 arrest and apoptosis in vitro . Excellent in vivo antitumor efficacy with 23 was achieved in an MV;411 mouse xenograft model. Pleasingly, compound 23 (hERG IC 50 > 30 μM) mitigated the inhibition of the human ether-à-go-go-related gene (hERG) ion channel compared with 10 (hERG IC 50 = 2.8 μM). This work provides a promising BD2-selective lead for the development of more effective and safe BET inhibitors as anticancer agents.
Organizational Affiliation:
Department of Medicinal Chemistry, School of Pharmacy, China Pharmaceutical University, Nanjing 211198, China.